Figure 1.
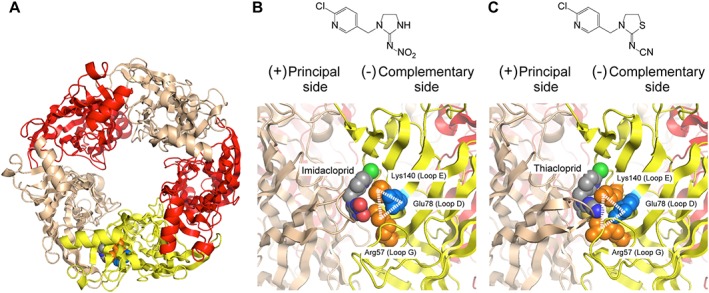
Homology models of the ligand binding domain of fruit fly Dα1/chicken β2 nACh receptor in complex with imidacloprid and thiacloprid. (A) Overall top view of the (Dα1)3(β2)2 nACh receptor model generated from human (α4)2(β2)3 nACh receptor docked with imidacloprid. The Dα1 subunits at principal and complementary sides are coloured tan and yellow, respectively, whereas the β2 subunits are coloured red. (B) Chemical structure of imidacloprid and close‐up view of the imidacloprid binding site. (C) Chemical structure of thiacloprid and close‐up view of the thiacloprid binding site. Arg57 and Lys140 interacted electrostatically with the nitro group of imidacloprid (B) and the cyano group of thiacloprid (C). Glu78 made salt bridges with Arg57 and Lys140 to form a ‘loop D‐E‐G triangle’ (B, C). In each panel, the main chains of the nACh receptors are drawn as cartoon, whereas Arg57 (coloured orange), Lys140 (coloured orange), Glu78 (coloured blue) and the neonicotinoids are drawn as space filling models. For neonicotinoids, carbon‐, nitrogen‐, oxygen‐, sulfur‐ and chlorine‐atoms are coloured grey, blue, red, tan and green, respectively.
