Figure 3.
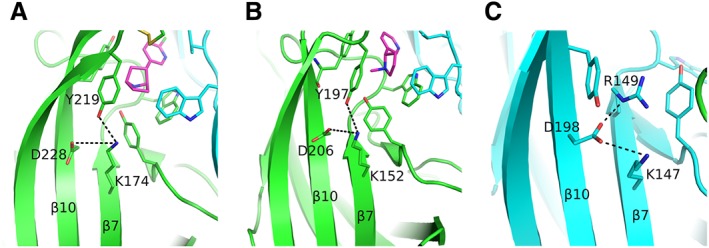
Rearrangements upon agonist binding. (A) The epibatidine‐bound α2 subunit showing the interaction of loop‐C Tyr219 with the β7‐strand Lys174, probably weakening the interaction between the residues of β7 and β10 strands. The (+) side is shown in green, the agonist in magenta and the (−) subunit in cyan. (B) Similarly for the α4 subunit bound to nicotine. Colours as in (A). (C) The β2‐subunit Asp198 on β10‐strand acquires a rotamer never observed before in α subunits. It is further stabilized by interactions with two positively charged residues of β7 strand. The β2 subunit is shown in cyan. α4 and β2 subunits were retrieved from PDB ID: 5KXI (Morales‐Perez et al., 2016) and α2 subunit from PDB ID: 5FJV (Kouvatsos et al., 2016). The coordinates of all the structures depicted were retrieved from Protein Data Bank (http://www.wwpdb.org), and PyMol (http://www.pymol.org) was used to generate the figures.
