Figure 3.
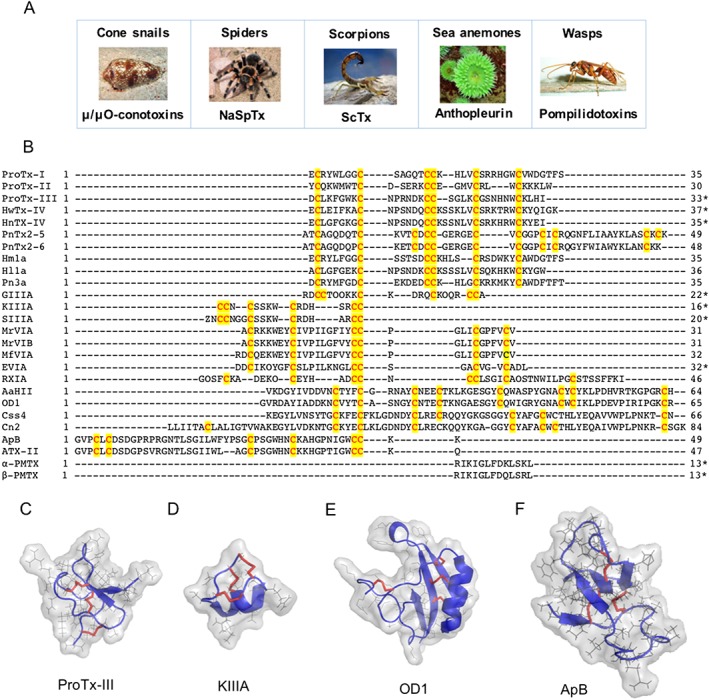
Natural peptide toxins targeting NaV channels. (A) μ/μO‐Conotoxins are a large group of peptidic toxins isolated from cone snails venoms (Lewis et al., 2012), NaSpTx is a large group of peptidic toxins isolated from spider venoms (Klint et al., 2012), ScTx is a group of toxins isolated from the venom of scorpions, anthopleurins are peptidic toxins isolated from sea anemone (Schweitz et al., 1981) and pompilidotoxins are peptides isolated from wasps (Konno et al., 1997; Konno et al., 1998). (B) Amino acid sequences alignment of the peptidic toxins discussed in this review, with highly conserved cysteines highlighted in red and yellow, and C‐terminal amidation denoted by an asterisk (*). Sequences were obtained from Arachnoserver, Conoserver and UniProt databases. (C–F) Structure of major classes of natural peptide toxins targeting NaV channels, including NaSpTx ProTx‐III isolated from the spider T. pruriens (Cardoso et al., 2015), μ‐conotoxin KIIIA isolated from Conus kinoshitai (Khoo et al., 2012), ScTx OD1 isolated from the scorpion Odonthobuthus doriae (Durek et al., 2013) and anthopleurin B (ApB) isolated from the sea anemone A. xanthogrammica (Monks et al., 1995). Disulfide bonds are represented as red sticks, amino acids side chains as grey lines and amino acids core chains as blue cartoon. Three‐dimensional structures were obtained from the RCSB Protein Data Bank and prepared in PyMol (DeLano, 2002).
