Abstract
Metastasis to distant organs is a particularly ominous feature of malignant cancer. LKB1 (also known as STK11) has been identified as a tumor suppressor in several types of cancers. Here, we show that LKB1 is at low levels and is negatively associated with poor clinical outcomes in pancreatic cancer (PC). LKB1 is inversely correlated with Snail protein in PC, in which the loss of LKB1 facilitates metastasis through elevating Snail protein level. Furthermore, LKB1 boosts Snail's interaction with E3 ligase FBXL14, leading to increasing ubiquitin‐mediated Snail degradation. Notably, metformin could increase Snail protein ubiquitination via augmenting the location of LKB1 at cytoplasm as well as increasing LKB1 expression. Altogether, our data established that LKB1 impedes invasion and metastasis by decreasing the Snail protein level in PC. Targeting the LKB1/FBXL14/Snail axis may represent a promising therapeutic strategy and metformin might be beneficial for PC therapy through activating the LKB1‐mediated Snail ubiquitination pathway.
Keywords: liver kinase B1, metformin, pancreatic cancer, Snail, ubiquitination
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC), one of the most fatal gastrointestinal tumors, is the major leadinzg cause of cancer‐related death worldwide. Pancreatic cancer (PC) accounts for approximately 7% of all cancer deaths in the USA.1 In the clinical setting, local or distant metastatic foci have been formed in most patients due to the poor technology for early detection for PC. The occult progression of this aggressive disease leads to the gloomy result that the overall 5‐year survival is approximately 8% and the median survival of all patients is still less than 6 months.1 Thus, to improve the survival and quality of PC patients’ life, it is important to explore the molecular mechanisms that can be beneficial for PC diagnosis or treatment.
Liver kinase B1 (LKB1), also known as serine‐threorine kinase 11 (STK11), has been identified as a critical tumor suppressor in several cancers.2, 3 Increasing evidence suggests that LKB1 is involved in regulating tumor progression through modulating the downstream pathways, such as AMPK/m‐TOR, CyclinD1, p21 and Src, or cooperating with other genes, such as K‐RAS and p53.2, 4, 5, 6, 7 Although many studies have been conducted on the biological functions of LKB1 in tumorigenesis, the underlying mechanisms are largely unknown. Snail, an important epithelial‐mesenchymal transition (EMT) inducer, is closely correlated with tumor metastasis.8 In PC, Snail promotes malignancy and metastasis by recruiting the E3 ligase Ring1B.9 In addition, Snail cooperation with KrasG12D facilitates pancreatic fibrosis and maintains the stemness of PC cells.9, 10 Therefore, Snail has been regarded as a potential target in pancreatic diagnosis and treatment. However, the correlation between LKB1 and Snail in PC needs to be characterized, which will be beneficial for exploring potential therapeutic strategies in clinic.
In this study, we demonstrate that the level of LKB1 negatively correlates with the Snail protein level both in PC samples and in PC cell lines. In addition, the opposite functions in PC metastasis between LKB1 and Snail also are detected. In vitro, LKB1 increases ubiquitination of Snail by promoting the interaction between Snail and E3 ligase FBXL14, resulting in Snail protein degradation and attenuation of PC cells’ migration and invasion. Furthermore, our data illustrate that metformin arrests PC cells’ migration and invasion through LKB1‐dependent Snail ubiquitination and degradation. Taken together, we believe that LKB1 might be a potent regulator of Snail for PC malignancy and metastasis, and metformin may be promising drug to be implemented in PC therapy.
2. MATERIALS AND METHODS
2.1. Tumor samples
Our study was approved by the Shanghai Institutes for Biological Science, Chinese Academy of Sciences. Two validated cohorts of patients who had provided informed written consent were used. One cohort consisted of 51 paired PC samples, which were obtained at the time of surgical resection from Changhai Hospital. The samples were used for real‐time PCR and western blot analyses. The experiments were repeated 3 times. The other cohort consisted of 51 PC patients, with specimens obtained as tissue microarrays (TMAs) from the Biobank Center of National Engineering Center for Biochip at Shanghai.
2.2. Immunological histological chemistry analysis
The immunological histological chemistry (IHC) analysis was performed as described previously.11 The IHC evaluation of protein expression intensity in PC tissues and normal adjacent tissues was performed independently by 2 pathologists from the department of Pathology, Zhongshan Hospital (Fudan University). The staining intensity was scored as described previously.12
The following primary antibodies were used: anti‐LKB1 antibody from Cell Signaling Technology and anti‐Snail from Thermo Fisher.
2.3. Cell culture
The PC cell lines (Capan‐1, Capan‐2, PANC‐1, Mia paca‐2, CFPAN‐1, BxPC‐3, HPAC, SW1990 and ASPC‐1) and HEK‐293T were obtained from the American Type Culture Collection (Manassas, VA, USA). The human pancreatic epithelial cell (HPDE) was obtained from the type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. 293‐GPG was kindly provided by Dr SK Muthuswamy from Cold Spring Harbor Laboratory. All of the above were cultured with DMEM medium (Life Technologies) supplemented with 10% FBS (Hyclone) and 1% penicillin/streptomycin (Life Technologies). For all cultures, cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
2.4. Mouse embryonic fibroblast extraction, culture and infection
The methods of mouse embryonic fibroblast (MEF) extraction and culture in vitro were performed as described previously.13 In our study, LKB1lox/lox mice (originally provided by Dr Hongbin Ji) were used to extract MEF. MEF cells cultured in 6‐well plates (20‐30% confluence) were infected with lentivirus carrying Cre recombinase (2 × 106p.f.u) for 4 hours and cultured for another 24‐48 hours to analyze the correlation between LKB1 and Snail. The experiments were repeated at least 3 times.
2.5. Nuclear and cytoplasmic protein extraction
Nuclear and cytoplasmic proteins were extracted as described previous.14 In brief, cells were treated with metformin or leptomycin B, respectively, for indicated times. Cells were harvested and lysed in ice‐cold cytoplasmic protein extraction regent (CER) I for 15 minutes, followed by addition of ice‐cold CER II and incubation on ice for another 1 minutes. Cell lysates then were centrifuged at 16 000 × g for 5 minutes at 4°C and the supernatant containing the cytoplasmic proteins was removed and stored for use. The insoluble pellet was resuspended in ice‐cold nuclear protein extraction regent (NER) and incubated on ice for 40 minutes. We centrifuged the tube at maximum speed for 10 minutes at 4°C and immediately transferred the supernatant fraction to a clean tube; extracts were stored at −20°C until use. The experiments were repeated at least 3 times.
2.6. Western blotting and immunoprecipitation
Western blotting and immunoprecipitation (IP) assays were extracted as described previously.15 The following primary antibodies were used: anti‐LKB1 antibody from Abmart (Cambridge, MA, USA); anti‐Hsp90, anti‐Snail, anti‐AMPK, anti‐Twist, anti‐Slug, anti‐GSK3β, anti‐p‐GSK3β, anti‐HA, anti‐Myc, anti‐flag antibodies and HRP‐conjugated secondary antibodies from Cell Signaling Technology (Boston, MA, USA); and anti‐FBXL14 antibody from ABclonal (Wuhan, China). The experiments were repeated at least 3 times.
2.7. RNA isolation and real‐time PCR
RNA isolation and real‐time PCR assays were performed as described previously.15 The primers used for real‐time PCR are shown in Table 1.
Table 1.
Sequences of primers used in real time‐qPCR
| Primer | Sequence |
|---|---|
| LKB1 F | 5′‐TGTCGGTGGGTATGGACAC‐3′ |
| LKB1 R | 5′‐CCTTGCCGTAAGAGCCTTCC‐3′ |
| Snail F | 5′‐CTCTTTCCTCGTCAGGAAGC‐3′ |
| Snail R | 5′‐GGCTGCTGGAAGGTAAACTC‐3′ |
| E‐cadherin F | 5′‐TGCCCAGAAAATGAAAAAGG‐3′ |
| E‐cadherin R | 5′‐CTGGGGTATTGGGGGCATC‐3′ |
| FBXL14 F | 5′‐TGCGCTCCTGTGACAACATC‐3′ |
| FBXL14 R | 5′‐TGGGCTATGTAAGCCAGACTC‐3′ |
| β‐TrCP F | 5′‐CCAGACTCTGCTTAAACCAAGAA‐3′ |
| β‐TrCP R | 5′‐GGGCACAATCATACTGGAAGTG‐3′ |
| AMPKα F | 5′‐CTGTAAGCATGGACGGGTTGA‐3′ |
| AMPKα R | 5′‐AAATCGGCTATCTTGGCATTCA‐3′ |
| β‐ACTIN F | 5′‐CCTGGCACCCAGCACAAT‐3′ |
| β‐ACTIN R | 5′‐GGGCGGGACTCGTCATAC‐3′ |
2.8. Transwell migration and invasion assays
Migration and invasion assays were performed as described previously.15 The experiments were repeated at least 3 times.
2.9. 3‐D cell culture
Capan‐2 and PANC‐1 cells were cultured on Matrigel as described previously.16 In our study, as indicated, cells were propagated for 5‐12 days. The experiments were repeated at least 3 times.
2.10. Oncomine data analysis
The Oncomine database was analyzed as previously described.17 Briefly, the gene was searched using the threshold values as follows: fold‐change of 2, P‐value of .05 and gene rank in the top 10% among all differentially expressed genes. All the datasets were listed and ordered by P‐value; for each published dataset, the values were linked to the graphical representations of the original microarray dataset. The unpaired Student's t test was used to calculate P‐values.
2.11. Statistical analysis
Data are presented as the mean ± SD from at least 3 independent experiments. Two‐tailed Student's t tests were conducted using Instat 5.0 (GraphPad). P‐values less than .05 were considered as significant differences.
3. RESULTS
3.1. Liver kinase B1 negatively correlated with Snail expression in pancreatic cancer
To identify the expression pattern of LKB1 in PC, the clinical PC gene expression datasets obtained from Oncomine database were analyzed.18 The results demonstrated that LKB1 mRNA was significantly downregulated in clinical PC samples (Pei Pancreas, N = 36; Badea Pancreas, N = 39; Segare Pancreas, N = 11) compared with its surrounding non‐cancerous samples (Pei Pancreas, N = 16; Badea Pancreas, N = 39; Segare Pancreas, N = 6) (Figure 1A and Figure S1A).19, 20, 21 In addition, analysis of the Buchholz Pancreas dataset22 showed that the LKB1 mRNA level was at a lower level in Grade PanIN‐3 (N = 10) and PDAC (N = 8) contrasted with normal tissues (N = 6) in the context of the proposed multistep PDAC progression model (Figure 1B, left panel). We further analyzed the data from the Ishkawa dataset23 and the data illustrated that LKB1 mRNA was dramatically reduced in more advanced PC (stage IVB, N = 3) than that in either normal tissues (N = 25) or relatively lower stage PC (stage 0, N = 3; stage I, N = 3; stage II, N = 2; stage IVA, N = 13) (Figure S1B). In addition, similar analysis from TCGA showed us that LKB1 was significantly downregulated in PC of Grade 3 (N = 5) compared with the lower grades, Grade 1 and Grade 2 (Grade 1‐2, N = 12) (Figure 1B, right panel). To further identify the expression pattern of LKB1 in PC, we analyzed the mRNA expression of LKB1 in 51 paired human PC samples. The results showed that in the majority of samples (28/51) LKB1 mRNA was downregulated more than 2‐fold in pancreatic tumor tissues compared with matched non‐cancerous parts (Figure 1C).
Figure 1.
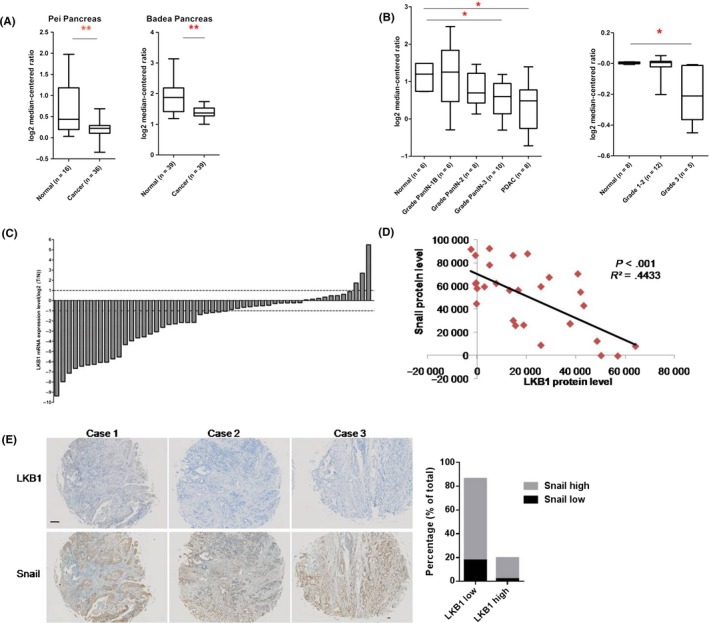
LKB1 is expressed at a lower level and negatively correlated with Snail expression in pancreatic cancer. A, Box plots comparing levels of LKB1 mRNA of different samples. **P < .01, Student's t test; B, mRNA levels of LKB1 in normal human pancreatic tissues and pancreatic cancers from different clinical grades or stages. **P < .01, *P < .05, Student’ s t test. Left, Buchholz Pancreas dataset; right, TCGA database; C, mRNA levels of LKB1 in 51 paired human pancreatic cancer tissues (T) and adjacent normal tissues (N). Data represent the mean ± SD (n = 3); D, The statistic result of western blot from pancreatic cancer samples; E, Immunological histological chemistry (IHC) staining for LKB1 and Snail in 3 representative pancreatic ductal adenocarcinoma (PDAC) cases. The plot presents the statistic result of IHC of 51 human PDAC samples. The definitions of “high” and “low” are given in the Methods section. Scale bar, 500 μm
Epithelial‐mesenchymal transition as one of the hallmarks of cancer has been involved in PC malignancy and metastasis.8 Interestingly, we found an inverse correlation between LKB1 and Snail protein in human PC tissues which were displayed in Figure 1C (Figure 1D). We further examined the expression patterns of LKB1 and Snail in another 51 human PDAC samples using tumor microarrays. Notably, IHC analyses revealed that almost 70% of human PDAC samples presented an LKB1low Snailhigh protein pattern (Figure 1E). Taken together, these results suggest that the LKB1, as a promisingly negative regulator of Snail protein, significantly correlates with PC malignancy and metastasis.
3.2. Liver kinase B1 deficiency promotes the migration and invasion via increasing Snail in pancreatic cancer cells
Mouse embryonic fibroblasts have been considered as an excellent model system to study the physiological or pathological consequences of selective gene ablations.24, 25, 26 Thus, MEF cells extracted from LKB1 lox/lox mice were cultured and subsequently infected with lentivirus carrying Cre recombinase to delete LKB1. The result showed that deficiency of LKB1 elevated the level of Snail in MEF cells (Figure 2A). Consistently, the protein levels of LKB1 and Snail were inversely correlated in 9 PC cell lines (Figure 2B) but had almost no significant correlation with other EMT inducers such as Slug and Twist (Figure S2A). Furthermore, continuously knocking down LKB1 in PANC‐1 or MIA paca‐2 cell lines (respectively named as PANC‐1 shLKB1 and MIA paca shLKB1) increased the Snail protein level; in contrast, forced expression of LKB1 in the other 5 PC cell lines including Capan‐2 cell (named Capan‐2 wtLKB1) resulted in a definite decrease in the pattern of Snail protein (Figure 2C and Figure S2B).
Figure 2.
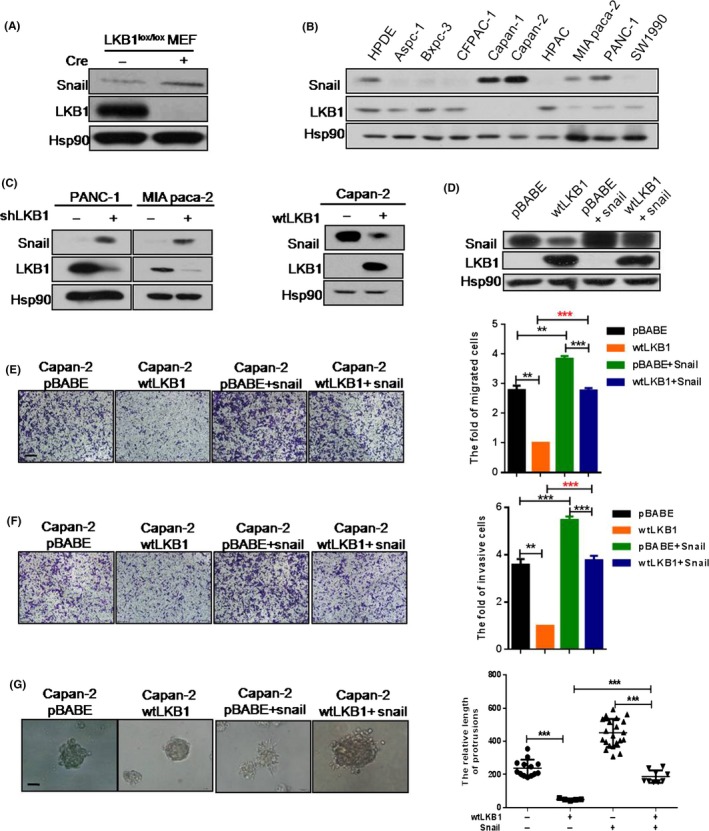
LKB1 deficiency promotes the migration and invasion via Snail in pancreatic cancer (PC) cells. A, Mouse embryonic fibroblast (MEF) cells extracted from LKB1lox/lox mice were infected and we analyzed the expression patterns of LKB1 and Snail; B, The protein levels of LKB1 and Snail; C, Snail and LKB1 protein expression were detected by western blot; D, Western blotting was performed to identity the overexpression of Snail in Capan‐2 wtLKB1 cell line; E‐G, Migration (E), invasion (F) transwell assays and 3‐D culture (G) were done in Capan‐2 pBABE, Capan‐2 wtLKB1 and Capan‐2 pBABE or Capan‐2 wtLKB1 with forced Snail expression cell lines. Quantitative results are illustrated, respectively, for panels E‐G. Data represent the mean ± SD (n = 3) from 3 separate experiments. **P < .01, ***P < .001, Student's t test. Scale bars: transwell assay, 200 μm; 3‐D culture, 50 μm
To define the potential effects of LKB1 deficiency in PC cell lines, we performed assays to evaluate the role of LKB1 in proliferation and malignancy. Our data showed that LKB1 had no notable effect on cell proliferation in PC cells (Figure S3A,B). However, loss of LKB1 prominently elevated migration and invasion in PANC‐1 (Figure S3C,E). Conversely, as the data show, stably overexpressing LKB1 significantly attenuated migration and invasion in Capan‐2 wtLKB1 cell lines when contrasted with control cells (Figure S3D,E,F). Given that 3‐D culture has been regarded as a powerful tool to investigate the molecular mechanism, we extended our 2‐D analyses to 3‐D culture. As the results show, in the 3‐D culture system LKB1‐silenced PANC‐1 cells formed far more protrusions and exhibited elevated invasive behavior in contrast to control cells (Figure S3G). Conversely, the morphology of cells tended to be spheres in Capan‐2 wtLKB1 compared with the control group (Figure S3H).
To investigate whether LKB1 regulates tumor metastasis through repression of Snail expression in PC cells, we restored the expression of Snail in the Capan‐2 wtLKB1 cell line (Figure 2D). As we supposed, Snail overexpression counteracted the changes of migration and invasion observed in 2‐D culture of Capan‐2 wtLKB1 cells (Figure 2E,F), as well as the increased protrusions and invasive behavior seen in 3‐D culturing of cells with LKB1 overexpression (Figure 2G). Thus, these results implied that Snail is a key downstream effector of decreased migration and invasion seen on LKB1 overexpression.
3.3. Liver kinase B1 suppresses Snail protein expression by ubiquitin‐mediated degradation pathway
To clarify the molecular mechanism of LKB1‐mediated Snail inhibition, first we found that Snail mRNA level was not significantly altered with knockdown of LKB1 in PC cells (Figure S4), suggesting that LKB1 may regulate Snail accumulation at a post‐transcriptional level. Following administration of epoxomicin, a specific proteasomal inhibitor,27 the protein level of Snail was restored in Capan‐2 wtLKB1 (Figure 3A). In addition, we found that LKB1 dramatically elevated the level of Snail ubiquitination (Figure 3B). Notably, the results from the time course of treatment in shLKB1 cells with cycloheximide, a protein synthesis inhibitor,28 indicated that loss of LKB1 increased the half‐life of Snail protein (PANC‐1 pLKO.1, 50 minutes; PANC‐1 wtLKB1, >240 minutes) (Figure 3C). In addition, epoxomicin also significantly improved the migration and invasion of Capan‐2 wtLKB1 (Figure 3D,E). Taken together, the data suggested that LKB1 might repress Snail accumulation through ubiquitin‐mediated degradation to inhibit PC migration and invasion.
Figure 3.
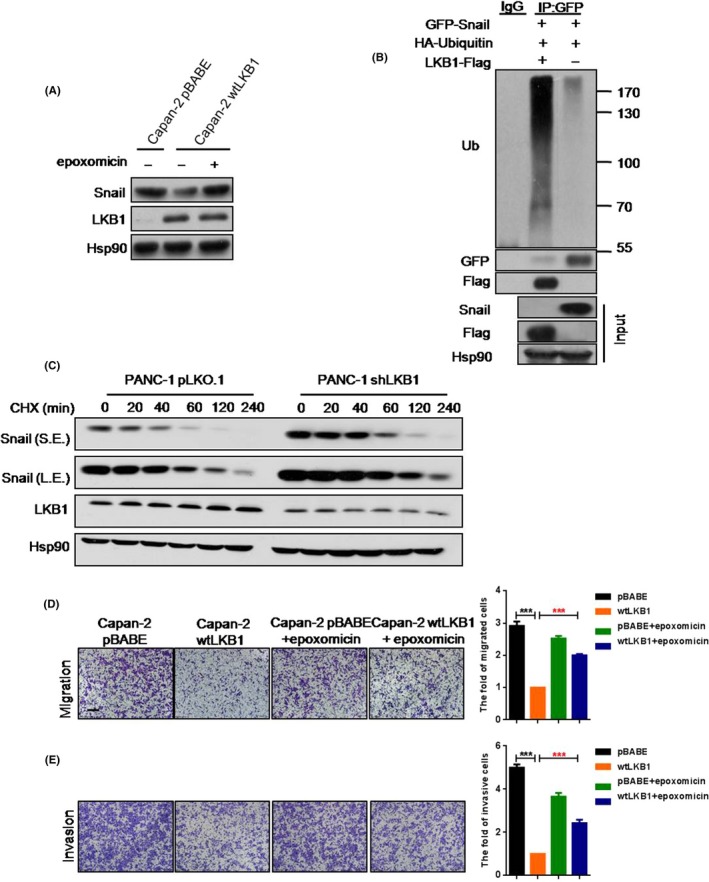
LKB1 suppresses Snail protein expression by ubiquitin‐mediated degradation pathway. A, The protein expression of Snail and LKB1 in Capan‐2 pBABE and Capan‐2 wtLKB1 cell lines which were treated with or without 10 μΜ epoxomicin for 2 h; B, The ubiquitination of Snail in 293T or 293T with forced expression of LKB1; C, Western blot to show the rates of Snail degradation in PANC‐1 pLKO.1 and PANC‐1 shLKB1 cell lines which were treated with CHX for indicated times (L.E., long exposure; S.E, short exposure); D,E Transwell assays were performed to show the migration (D) and invasion (E) of Capan‐2 pBABE, Capan‐2 wtLKB1 and Capan‐2 pBABE or Capan‐2 wtLKB1 with 10 μΜ epoxomicin treatment. Migration, 5 × 104 cells/well; invasion, 9 × 104 cells/well. Quantitative results are illustrated for panels (right). Data represent the mean ± SD (n = 3) from 3 separate experiments. **P < .01, ***P < .001, Student's t test. Scale bar, 200 μm
3.4. Liver kinase B1 promotes Snail degradation through enhancing the binding of Snail and E3 ligase FBXL14
Prior work suggested that activation of the GSK3β/βTrCp pathway resulted in Snail reduction,29 which led us to examine whether LKB1 suppressed Snail expression though the GSK3β/βTrCp pathway in PC cells. However, LKB1 overexpression in PANC‐1 or Capan‐2 cells did not lead to activation of GSK3β (Figure S5A,B). In addition, the protein level of snail was not altered in Capan‐2 wtLKB1 cells along with β‐TrCp siRNA (Figure S5C). Furthermore, we tested whether LKB1 mediated Snail degradation by AMPK, a key downstream kinase of LKB1.6, 7, 30 We found that the Snail protein level was not changed in Capan‐2 wtLKB1 cells with knockdown of AMPK by siRNA (Figure S5D), suggesting the existence of alternative mechanisms in regulating Snail gene expression by LKB1. Some studies have demonstrated that Snail degradation could be induced by E3 ligase FBXL14, which is independent of GSK3‐β/β‐TrCp pathway.31 Interestingly, the LKB1‐mediated Snail inhibition was blocked via FBXL14 deficiency silenced by siRNA in Capan‐2 (Figure 4A). In addition, loss of FBXL14 antagonized LKB1's repressive effect on migration and invasion in Capan‐2 (Figure 4B,C). To further investigate the relationship between LKB1 and Snail‐FBXL14 complex, we performed an IP assay to detect whether LKB1 directly regulates the interaction between Snail and FBXL14. Indeed, LKB1 had an interaction with both Snail and FBXL14 (Figure 4D,E and Figure S6A‐C) and forced expression of LKB1 dramatically enhanced FBXL14 binding to Snail (Figure 4F). These results suggested that LKB1 might recruit FBXL14 to Snail, which formed a protein complex LKB1/Snail/FBXL14 to facilitate the ubiquitination of Snail. Therefore, the data demonstrated that LKB1 promotes ubiquitin‐mediated Snail degradation through enhancing the binding of Snail and FBXL14.
Figure 4.
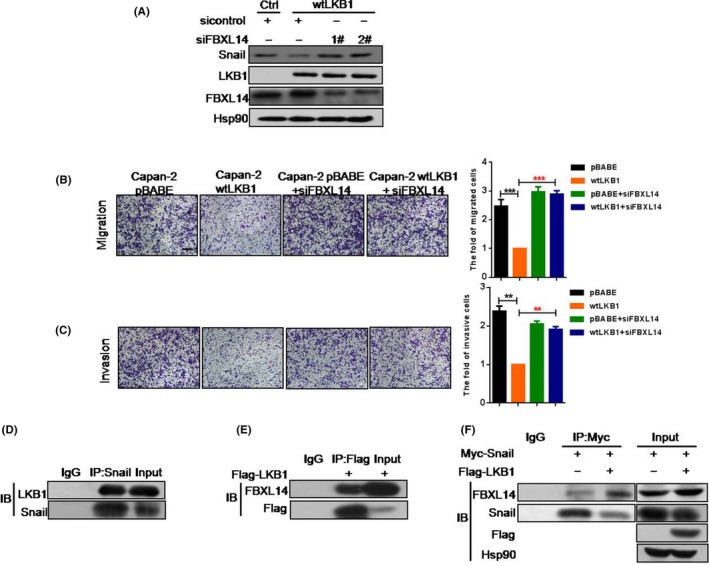
LKB1 promotes Snail degradation through enhancing the binding of Snail and E3 ligase FBXL14. A, Protein expression of Snail in Capan‐2 pBABE transfected with siControl, Capan‐2 wtLKB1 and Capan‐2 wtLKB1, respectively, transfected with siControl and siFBXL14; B,C, Transwell assays were employed to show the migration (B) and invasion (C) of Capan‐2 pBABE,, Capan‐2 wtLKB1 and Capan‐2 pBABE or Capan‐2 wtLKB1 transfected with FBXL14 siRNA for 24 h. Quantitative results are illustrated for panels (right). Data represent the mean ± SD (n = 3) from 3 separate experiments. **P < .01, ***P < .001, Student's t test. Scale bar, 200 μm; D, Immunoprecipitation (IP) analysis to show the interaction between endogenous LKB1 and Snail in PANC‐1; E, Western blot analysis to show the binding between LKB1 and endogenous FBXL14 in PANC‐1; F, Western blot analysis was performed to show the effect of LKB1 on interaction between Snail and endogenous FBXL14
3.5. Metformin suppresses migration and invasion through repressing Snail protein expression in liver kinase B1‐dependent pathway in pancreatic cancer cells
Epidemiological studies indicated that metformin might improve the survival rate of patients with PC.32 In our study, we demonstrated that metformin could attenuate Snail protein level in Capan‐2 wtLKB1 cell line in dose‐dependent or time‐dependent ways (Figure 5A,B). Notably, the LKB1 expression level was increased by metformin in Capan‐2 wtLKB1 cell line, whereas it played a negligible role in Capan‐2 cells, in which LKB1 is undetectable. Consistently, metformin not only inhibited Snail protein expression but also elevated the LKB1 level in PANC‐1 and PANC‐1 shLKB1 cell lines (Figure 5C). These results suggested that metformin‐stimulated Snail protein suppression might be mediated by LKB1 upregulation.
Figure 5.
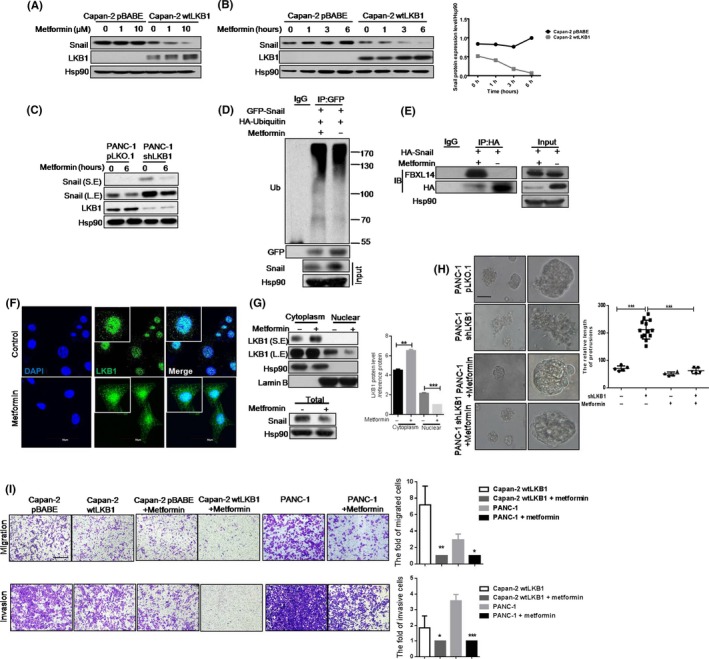
Metformin suppresses migration and invasion by repressing Snail protein expression in LKB1‐dependent pathway in pancreatic cancer (PC) cells; A, B, Snail and LKB1 protein expression in Capan‐2 wtLKB1 and the control cell line. Cells were treated with metformin for 6 h (A) or 10 μmol/L metformin for the indicated time (B). The plot showed the relative ratio of Snial/Hsp90 in panel B; C, Snail and LKB1 protein expression in PANC‐1 shLKB1 and the control cell line. Cells were treated with 10 μmol/L metformin for the indicated time; D, Snail ubiquitination was analyzed by immunoprecipitation (IP) assay in HEK‐293T cells with or without 10 μmol/L metformin treatment; E, IP assay was conducted to detect the interaction between Snail and FBXL14 in HEK‐293T with or without 10 μmol/L metformin treatment; F, LKB1 location with or without 10 μmol/L metformin treatment in Capan‐2 wtLKB1 cell line. Scale bar, 50 μm; G, LKB1 protein expression in nuclear or cytoplasm in Capan‐2 wtLKB1 cell line with or without metformin treatment. Quantitative results are illustrated for panels (right). Data represent the mean ± SD (n = 3) from 3 separate experiments. **P < .01, ***P < .001, Student's t test; H, The acinar morphologies of PANC‐1 shLKB1 and the control cell line in 3‐D culture with or without metformin treatment. Quantitative results are illustrated for panels (right). Data represent the mean ± SD (n = 3) from 3 separate experiments. ***P < .001, Student's t test; I, Transwell assays were used to show the migration (5 × 104 cells/well) and invasion (9 × 104 cells/well) of Capan‐2 pBABE (or Capan‐2wtLKB1) and Capan‐2 pBABE (or Capan‐2 wtLKB1) with 10 μmol/L metformin treatment for 24 h. Quantitative results are illustrated for panels (right). Data represent the mean ± SD (n = 3) from 3 separate experiments. ***P < .001, **P < .01, *P < .05, Student's t test. Scale bars: transwell assay, 200 μm; 3‐D culture, 50 μm
To address whether metformin suppresses the Snail protein level via LKB1‐mediated ubiquitination, IP assays showed that metformin enhanced the association between Snail and FBXL14, and promoted Snail ubiquitination (Figure 5D,E). Furthermore, we found that metformin increased the total LKB1 protein level and cytoplasmic LKB1 location (Figure 5G), which was consistent with the result from IF assay (Figure 5F). In addition, Snail protein was dramatically decreased by metformin in Capan‐2 wtLKB1 cell line (Figure 5G). Next, to identify the role of cytoplasmic LKB1 in Snail level, Leptomycin B, an inhibitor of nuclear export, was used to decrease LKB1's location in cytoplasm, and the data demonstrated that LKB1 was limited to nuclear and Snail protein level was increased (Figure S7A,B). Therefore, our result confirmed that the effect of metaformin on translocation of LKB1 to cytoplasm is responsible for Snail degradation.
To define the biological function of metformin in LKB1‐Snail regulation, we employed transwell assays. The results showed that metformin attenuated migration and invasion of Capan‐2 wtLKB1 and PANC‐1 cell lines, as well as inhibited protrusion formation and malignant phenotype in 3‐D culture (Figure 5H,I). Altogether, our data illustrated that metformin inhibits migratory and invasive behaviors and decreases Snail protein level, which is dependent on LKB1 level and location in PC cells.
4. DISCUSSION
As reported here, the lower expression of LKB1 is associated with high grade and advanced stage, and decreased LKB1 levels are related to poorer clinical outcome in PC patients. LKB1 protein level is negatively correlated with Snail protein level in PC cells and PC tissues. We further demonstrated that LKB1 promotes Snail protein degradation though enhancing interaction between E3 ligase FBXL14 and Snail. Our data showed that loss of LKB1 in PC cells significantly improved cell migration and invasion, which is mediated by elevating Snail protein level. Notably, metformin inhibits migration and invasion by increasing the LKB1 expression level and ubiquitin‐mediated degradation of Snail in PC cells. In summary, our studies uncover a new mechanism of metformin in the prevention and management of pancreatic malignancies.
Liver kinase B1 deficiency has frequently been linked to more malignant cancer behaviors, including invasion and metastasis.3, 5, 6 Snail is involved in the acquisition of invasive and migratory properties during tumourgenesis.9, 10 It is reported that scaffolding protein DIXDC1 suppresses Snail level in LKB1/MARK1‐positive cancer cells.33 Our studies demonstrate the negative relationship between LKB1 protein and Snail protein in PC samples and PC cell lines. However, we did not find a correlation between LKB1 and DIXDX1 in PC cell lines (Figure S5E). In addition, our observations suggest that LKB1 inhibits Snail protein level but not mRNA in PC but has no effect on the other EMT inducers (such as Slug, Twist). Furthermore, the expression of E‐cadherin, which is suppressed by Snail, has positively correlation with LKB1 in PC cells (Figure S2C).
FBXL14, belonging to the F‐box family, mainly localizes at cytoplasm and has important roles in regulating EMT inducers (such as Snail, Slug and Twist).34, 35 Previous data demonstrated that hypoxia‐controlled FBXL14 improves the Snail protein level through regulating proteasome‐mediated Snail degradation.31 In this study, we demonstrate that LKB1 increases the interaction between FBXL14 and Snail and promotes FBXL14‐mediated Snail protein degradation though AMPK‐independent signaling. In addition, we observed that FBXL14 mRNA (Figure S6C) and protein levels were increased when we introduced full‐length LKB1 into PC cells. LKB1, as a key kinase in energy metabolism, is activated to antagonize cell injury and is involved in an energy stress condition.36, 37 Depletion of LKB1 enhances the effects of hypoxia inducible factor‐1α (HIF‐1α) on metabolism reprogramming to maintain cell proliferation in cancer.38 Thus, we consider whether the upregulation of the FBXL14 level was associated with the modulation of LKB1/HIF‐1α signaling. The specific mechanism needs to be further explored.
Metformin has received tremendous attention due to its potential anti‐cancer effects.32, 39 Indeed, we observed that metformin inhibits migration and invasion in PC cells. Ongoing increasing evidence has illustrated that the ability of metformin to repress tumorigenesis depends on AMPK‐dependent or AMPK–independent pathway.40, 41 Recent reports have suggested that metformin treatment decreases EMT markers level including Snail in PC from obese mice.42 In our study, we demonstrated that metformin decreases Snail protein in a dose‐dependent and time‐dependent manner in Capan‐2 wtLKB1 cell line as well as in PANC‐1 cell line. Furthermore, we identified that LKB1 protein level is notably elevated by metformin in PC cells. These results suggest that the impact of metformin on suppressing migration and invasion might be dependent on LKB1‐mediated Snail protein degradation in PC. Interestingly, metformin facilitates Snail ubiquitination through elevating the linkage between Snail and E3 ligase FBXL14, which might be mediated by LKB1. Notably, downregulated Snail is partly correlated with high LKB1 protein level. TAM analyses showed us that approximately 70% of PC samples (35/51) exhibited LKB1lowSnailhigh expression pattern and less than 20% of PC samples (9/51) showed LKB1highSnailhigh protein pattern. Therefore, clinical data supported that expression of LKB1 is negatively correlated with high Snail expression in PC tissues. In addition, metformin increased LKB1 expression and translocation to cytoplasm. Our study provides new insight into how LKB1 serves as an important negative regulator by suppressing Snail protein in the pathogenesis of PC. Targeting of LKB1 with metformin could offer a promising therapeutic strategy for PC.
In summary, our analyses have illustrated that LKB1 is a potential suppressor of metastasis of PC cells. We further demonstrated that LKB1 promotes Snail protein degradation though enhancing interaction between E3 ligase FBXL14 and Snail to increase Snail ubiquitination. In addition, we identified that metformin can increase the LKB1 protein level and the interaction between E3 ligase FBXL14 and Snail in PC cells, which might be independent of AMPK or GSK3β pathway. These findings have improved our understanding of specific functions and mechanisms of LKB1 in PC. Moreover, metformin, as a sensitizer or combined with first‐line chemotherapeutic agents, may provide a promising therapeutic strategy for PC.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
ACKNOWLEDGMENTS
We thank Dr Hongbin Ji (SIBCB, CAS, China) for the LKB1lox/lox mice and plasmids pLKO.1‐shLKB1 408 and pLKO.1‐shLKB1 409. We thank Dr Chenghao Shao (The Second Military Medical University) for the pancreatic cancer patients’ samples.
Song L, Guo J, Chang R, et al. LKB1 obliterates Snail stability and inhibits pancreatic cancer metastasis in response to metformin treatment. Cancer Sci. 2018;109:1382–1392. https://doi.org/10.1111/cas.13591
Funding informationNational Key R&D Program of China (2017YFC1600104); the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12010100); National Natural Science Foundation of China (81672956, 81672892, 81370571).
Song and Guo are contributed equally to this work.
Contributor Information
Xianbao Zhan, Email: zhanxinbao@126.com.
Lixing Zhan, Email: lxzhan@sibs.ac.cn.
REFERENCES
- 1. American Cancer Society . Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018. [Google Scholar]
- 2. Korsse SE, Peppelenbosch MP, van Veelen W. Targeting LKB1 signaling in cancer. Biochim Biophys Acta. 2013;1835:194‐210. [DOI] [PubMed] [Google Scholar]
- 3. Boudeau J, Sapkota G, Alessi DR. LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett. 2003;546:159‐165. [DOI] [PubMed] [Google Scholar]
- 4. Tiainen M, Vaahtomeri K, Ylikorkala A, Makela TP. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1). Hum Mol Genet. 2002;11:1497‐1504. [DOI] [PubMed] [Google Scholar]
- 5. Scott KD, Nath‐Sain S, Agnew MD, Marignani PA. LKB1 catalytically deficient mutants enhance cyclin D1 expression. Cancer Res. 2007;67:5622‐5627. [DOI] [PubMed] [Google Scholar]
- 6. Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91‐99. [DOI] [PubMed] [Google Scholar]
- 7. Shackelford DB, Shaw RJ. The LKB1‐AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shields MA, Ebine K, Sahai V, Munshi HG. Snail cooperates with KrasG12D to promote pancreatic fibrosis. Mol Cancer Res. 2013;11:1078‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou W, Lv R, Qi W, et al. Snail contributes to the maintenance of stem cell‐like phenotype cells in human pancreatic cancer. PLoS ONE. 2014;9:e87409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu HT, Yu H, Zhang XY, Gao HJ, et al. UNC51‐like kinase 1 as a potential prognostic biomarker for hepatocellular carcinoma. Int J Clin Exp Pathol. 2013;6:711‐717. [PMC free article] [PubMed] [Google Scholar]
- 12. Pencheva N, Tran H, Buss C, Tavazoie SF, et al. Convergent Multi‐miRNA targeting of apoE drives LRP1/LRP8‐dependent melanoma metastasis and angiogenesis. Cell. 2012;151:1068‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saeed H, Taipaleenmäki H, Aldahmash AM, Abdallah BM, Kassem M. Mouse embryonic fibroblasts (MEF) exhibit a similar butnot identical phenotype to bone marrow stromal stem cells (BMSC). Cell Rev. 2012;8:318‐328. [DOI] [PubMed] [Google Scholar]
- 14. Zhou Y, Chang RX, Ji WW, Zhan LX, et al. Loss of scribble promotes snail translation through translocation of HuR and enhances cancer drug resistance. J Biol Chem. 2016;291:291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu Y, Chang R, Peng Z, et al. Loss of polarity protein AF6 promotes pancreatic cancer metastasis by inducing Snail expression. Nat Commun. 2015;6:7184. [DOI] [PubMed] [Google Scholar]
- 16. Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF‐10A mammary epithelial acini grown in three‐dimensional basement membrane cultures. Methods. 2003;30:256‐268. [DOI] [PubMed] [Google Scholar]
- 17. Chen D, Sun Y, Wei Y, Zhang P, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo‐YAP pathway and a prognostic marker. Nat Med. 2012;18:1511‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data‐mining platform. Neoplasia. 2004;6:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pei H, Li L, Fridley BL, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating AKT. Cancer Cell. 2009;16:259‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole‐tissue and microdissected pancreatic ductaldenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016‐2027. [PubMed] [Google Scholar]
- 21. Segara D, Biankin AV, Kench JG, et al. Expression of HOXB2: a retino acidsignaling target in pancreatic cancer and pancreatic intraepithelial neoplasia. Clin Cancer Res. 2005;9:3587‐3596. [DOI] [PubMed] [Google Scholar]
- 22. Buchholz M, Braun M, Heidenblut A, et al. Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene. 2005;24:6626‐6636. [DOI] [PubMed] [Google Scholar]
- 23. Ishikawa M, Yoshida K, Yamashita Y, Ota J, Takada S, Mano H. Experimental trial for diagnosis of pancreatic ductal carcinoma based on gene expression profiles of pancreatic ductal cells. Cancer Sci. 2005;96:387‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lengner CJ, Lepper C, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Primary mouse embryonic fibroblasts: a model of mesenchymal cartilage formation. J Cell Physiol. 2004;200:327‐333. [DOI] [PubMed] [Google Scholar]
- 25. Steinman HA, Sluss HK, Sands AT, Pihan G, Jones SN. Absence of p21 partially rescues Mdm4 loss and uncovers an antiproliferative effect of Mdm4 on cell growth. Oncogene. 2004;23:303‐306. [DOI] [PubMed] [Google Scholar]
- 26. Lowe SW, Jacks T, Housman DE, Ruley HE. Abrogation of oncogene‐associated apoptosis allows transformation of p53‐deficient cells. Proc Natl Acad Sci USA. 1994;91:2026‐2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci USA. 1999;96:10403‐10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kreuz S, Siegmund D, Scheurich P, Wajant H. NF‐kappa B inducers upregulate cFLIP, a cycloheximide‐sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964‐3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou BHP, Deng J, Xia WY, et al. Dual regulation of Snail by GSK‐3 beta‐mediated phosphorylation in control of epithelial‐mesenchymal transition. Nat Cell Biol. 2004;6:931. [DOI] [PubMed] [Google Scholar]
- 30. Fu A, Screaton RA. Using kinomics to delineate signaling pathways control of CRTC2/TORC2 by the AMPK family. Cell Cycle. 2008;7:3823‐3828. [DOI] [PubMed] [Google Scholar]
- 31. Vinas‐Castells R, Beltran M, Valls G, et al. The hypoxia‐controlled FBXL14 ubiquitin ligase targets snail1 for proteasome degradation. J Biol Chem. 2010;285:3794‐3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Magruder JT, Elahi D, Andersen DK. Diabetes and pancreatic cancer: chicken or egg? Pancreas. 2011;40:339‐351. [DOI] [PubMed] [Google Scholar]
- 33. Goodwin JM, Svensson RU, Lou HJ, Winslow MM, Turk BE, Shaw RJ. An AMPK‐independent signaling pathway downstream of the LKB1 tumor suppressor controls Snail1 and metastatic potential. Mol Cell. 2014;55:436‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Das T, Purkayastha‐Mukherjee C, D'Angelo J, Weir M. A conserved F‐box gene with unusual transcript localization. Dev Genes Evol. 2002;212:134‐140. [DOI] [PubMed] [Google Scholar]
- 35. Vernon AE, LaBonne C. Slug stability is dynamically regulated during neural crest development by the F‐box protein Ppa. Development. 2006;133:3359‐3370. [DOI] [PubMed] [Google Scholar]
- 36. Lander R, Nordin K, LaBonne C. The F‐box protein Ppa is a common regulator of core EMT factors Twist, Snail, Slug, and Sip1. J Cell Biol. 2011;194:17‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van der Velden YU, Wang LQ, Clevers H, et al. The serine‐threonine kinase LKB1 is essential for survival under energetic stress in zebrafish. Proc Natl Acad Sci U S A. 2011;108:4358‐4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolff NC, Vega‐Rubin‐de‐Celis S, Brugarolas J, et al. Cell‐type‐dependent regulation of mTORC1 by REDD1 and the tumor suppressors TSC1/TSC2 and LKB1 in response to hypoxia. Mol Cell Biol. 2011;31:1870‐1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morales DR, Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med. 2015;66:17‐29. [DOI] [PubMed] [Google Scholar]
- 40. Faubert B, Vincent EE, Griss T, Jones RG, et al. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF‐1 alpha. Proc Natl Acad Sci U S A. 2014;111:2554‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bhaw‐Luximon A, Jhurry D. Metformin in pancreatic cancer treatment: from clinical trials through basic research to biomarker quantification. J Cancer Res Clin Oncol. 2016;142:2159‐2171. [DOI] [PubMed] [Google Scholar]
- 42. Cifarelli V, Lashinger LM, Devlin KL, Hursting SD, et al. Metformin and rapamycin reduce pancreatic cancer growth in obese prediabetic mice by distinct microRNA‐regulated mechanisms. Diabetes. 2015;64:1632‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


