Figure 1.
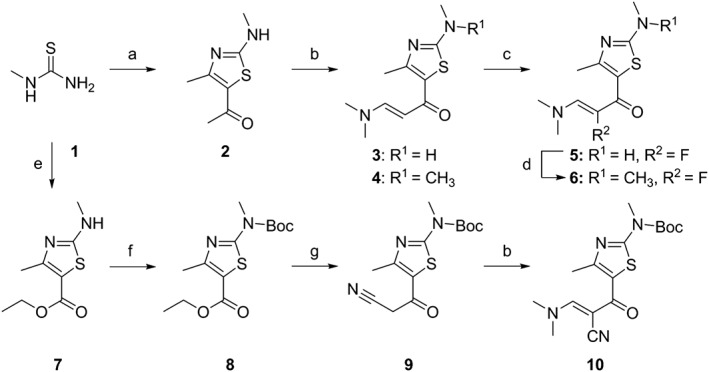
Synthetic scheme for enaminones 3–6 and 10. Reagents and conditions: (a) 3‐chloro‐2,4‐pentadione, C5H5N, MeOH, room temperature, overnight, 53%; (b) DMF‐DMA, reflux, 8 h, 3: 21%, 10: 62%, or microwave, 150°C, 1 h, 4: 38%; (c) Selectfluor, MeOH, ice bath, 1 h, 30%; (d) MeI, NaH, THF, 40°C, 3.5 h, 82%; (e) ethyl‐2‐chloroacetoacetate, DMAP, DCM, room temperature,4 h, 85%; (f) di‐tert‐butyl dicarbonate, DCM, Et3N, 8 h, room temperature, 93%; (g) LiNiPr2, MeCN, −78°C, 10 min; overnight, HCl, H2O, 48%.
