Abstract
We previously reported a phase I clinical trial of a peptide vaccine ring finger protein 43 (RNF43) and 34‐kDa translocase of the outer mitochondrial membrane (TOMM34) combined with uracil‐tegafur (UFT)/LV for patients with metastatic colorectal cancer (CRC), and demonstrated the safety and immunological responsiveness of this combination therapy. In this study, we evaluated vaccination‐induced immune responses to clarify the survival benefit of the combination therapy as adjuvant treatment. We enrolled 44 patients initially in an HLA‐masked fashion. After the disclosure of HLA, 28 patients were in the HLA‐A*2402‐matched and 16 were in the unmatched group. In the HLA‐matched group, 14 patients had positive CTL responses specific for the RNF43 and/or TOMM34 peptides after 2 cycles of treatment and 9 had negative responses; in the HLA‐unmatched group, 10 CTL responses were positive and 2 negative. In the HLA‐matched group, 3‐year relapse‐free survival (RFS) was significantly better in the positive CTL subgroup than in the negative‐response subgroup. Patients with negative vaccination‐induced CTL responses showed a significant trend towards shorter RFS than those with positive responses. Moreover, in the HLA‐unmatched group, the positive CTL response subgroup showed an equally good 3‐year RFS as in the HLA‐matched group. In conclusion, vaccination‐induced CTL response to peptide vaccination could predict survival in the adjuvant setting for stage III CRC.
Keywords: adjuvant chemotherapy, clinical trial, colorectal cancer, cytotoxic T lymphocytes, peptide vaccine therapy
1. INTRODUCTION
Adjuvant chemotherapy for colorectal cancer (CRC) is usually performed for patients with stage III CRC who have undergone R0 resection, to prevent recurrence and improve their prognoses.1 Several studies in Western countries have shown that adding oxaliplatin (L‐OHP) to 5‐fluorouracil (FU)/leucovorin (LV) or capecitabine improves adjuvant treatment of colon cancer.2, 3 In Japan, however, some specialists are skeptical of L‐OHP as an adjuvant treatment for stage III colon cancer because of better outcomes in Japanese randomized trials than those in Western countries and cumulative neurotoxicity risk with L‐OHP‐based therapy.4, 5, 6
We previously reported a phase I clinical trial of a peptide vaccine ring finger protein 43 (RNF43) and 34‐kDa translocase of the outer mitochondrial membrane (TOMM34) combined with uracil‐tegafur (UFT)/LV for patients with metastatic CRC, and demonstrated the safety and immunological responsiveness of this combination therapy.7, 8, 9 In this study, we evaluated vaccination‐induced immune responses to clarify the survival benefit of a peptide vaccine combined with UFT/LV as adjuvant treatment for selected patients with stage III CRC.
2. MATERIALS AND METHODS
2.1. Patients and eligible criteria
Patients were eligible for enrollment if they were 20‐80 years old with histologically confirmed stage III CRC, had adequate critical organ functions, and had an ECOG performance status of 0 or 1. Patients were excluded if they were pregnant, breastfeeding, were trying to become pregnant, had an active infectious disease, had multiple cancers, or took steroids or immunosuppressive therapy. The study was carried out in accordance with the Declaration of Helsinki on experimentation with human subjects, and was approved by the Institutional Ethical Review Boards of Kindai University (Approval No. 20‐110) and Yamaguchi University School of Medicine (approval no. H22‐175). The study was registered at the UMIN Clinical Trials Registry as UMIN000003552 (http://www.umin.ac.jp/ctr/index.htm). Written informed consent was obtained from all patients at the time of enrollment.
2.2. Peptides and drugs
HLA‐A*2402‐restricted RNF43 (NSQPVWLCL) and TOMM34 (KLRQEVKQNL) peptides were synthesized by the American Peptide Company (Sunnyvale, CA, USA) according to a standard solid‐phase synthesis method; preclinical trials previously confirmed that the peptides did not produce acute toxicity.8 UFT is a relatively old oral fluoropyrimidine that was developed in Japan in the 1980s. It has many indications for metastatic and advanced solid cancers, including those of the colon, lung, breast, and pancreas, and gastric cancer.10 In metastatic CRC, UFT/LV was demonstrated to have the same clinical efficacy as 5‐FU/LV and comparable pharmacokinetics between Japanese and US patients.11, 12, 13
2.3. Study design
Detailed information of the study design was described previously.9 Briefly, this was a phase II, non‐randomized, single‐arm study in which the HLA‐A status was double‐blinded. The therapy consisted of a cocktail of 2 epitope peptides with UFT/LV. The HLA‐A status of all enrolled patients was double‐blinded, and each patient received the same regimen of a peptide cocktail and UFT/LV chemotherapy. The cocktail of 2 peptides (1 mg of each peptide) was subcutaneously administered to patients once every 7 days, 5 times. All patients also received daily oral doses of UFT (300 mg/m2/d) plus LV (UZEL: 75 mg/d) for 28 days. Each cycle of treatment was followed by 1 week of rest. Patients received 6 cycles of treatment unless their disease relapsed. The primary objective was the comparison of relapse‐free survival (RFS) between patients with HLA‐A*2402 vs those without HLA‐A*2402. Secondary objectives included comparisons between the 2 groups regarding overall survival (OS), safety, tolerability and peptide‐specific activities of cytotoxic T lymphocytes (CTL).
2.4. Enzyme‐linked immunospot assay
Peptide‐specific CTL responses were estimated by the in vitro enzyme‐linked immunospot (ELISPOT) assay as previously described.14 Briefly, frozen peripheral blood mononuclear cells (PBMC) from each patient were thawed simultaneously. PBMC (5 × 105/mL) were then cultured with 10 μg/mL of each peptide and 100 IU/mL of interleukin‐2 (Novartis, Emeryville, CA, USA) at 37°C for 2 weeks. Peptides were added to the cultures on Day 0 and Day 7. Following CD4 + cell depletion by the Dynal CD4‐Positive Isolation Kit (Invitrogen, Carlsbad, CA, USA), interferon (IFN)‐γ ELISPOT assays were performed using the Human IFN‐γ ELISpot PLUS Kit (MabTech, Nacka Strand, Sweden), according to the manufacturer's instructions. The positivity of antigen‐specific T cell response was quantitatively defined according to the evaluation tree algorithm.15 Briefly, the peptide‐specific T cell responses were classified into 4 grades (−, +, ++ and +++) depending on the peptide‐specific spots at different responder/stimulator ratios. Representative CTL‐positive data from ELISPOT assays are shown (Figure 1). We judged specimens to be positive when the algorithm indicated +, ++ or +++.
Figure 1.
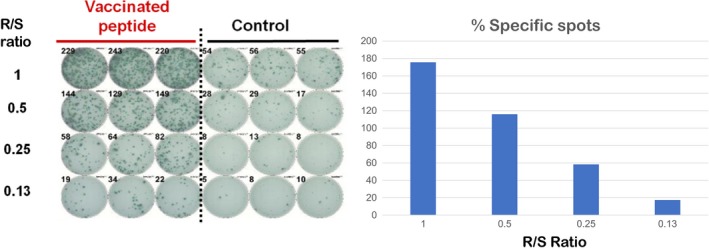
Enzyme‐linked immunospot assays detecting vaccinated peptides specific T cell activity. R/S, responder/stimulator
Among patients whose pre‐therapy CTL responses specific for RNF43/TOMM34 were −/−, −/+ or +/−, those whose responses after 2 cycles of therapy were −/− were considered to have negative responses. Among patients whose pre‐therapy CTL responses specific for RNF43/TOMM34 were −/+ or +/−, those who had a positive response to either component after 2 cycles of treatment were considered to have intermediate responses. Patients whose CTL responses specific for one or both peptides after 2 cycles of treatment were enhanced in comparison with their pre‐therapy levels were considered to have positive responses.
2.5. Statistical analyses
Survival curves were plotted using the Kaplan‐Meier method and compared with the log‐rank test. Survival was measured from the first vaccination until recurrence, death or the last follow‐up. Tests were always 2‐sided; P < .05 was considered significant. Statistical analyses were performed using JMP 11 software (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Demographics
The patient flow diagram is shown in Figure 2. Between December 2009 and December 2014, a total of 46 patients were enrolled in the study. Among the 46 patients, 44 received peptide vaccine therapy with UFT/LV (Figure 1). Two patients were excluded because they withdrew consent. All patients’ HLA‐A status was double‐blinded, and they all received the same regimen of peptide cocktail and UFT/LV chemotherapy. After the disclosure of HLA status, 28 patients had at least 1 HLA‐A*2402 allele, and 16 had no HLA‐A*2402 allele. The HLA‐A*2402‐matched and HLA‐A*2402‐unmatched groups did not significantly differ in sex, age, location of the primary tumor, dose of vaccine peptides administered or number of positive lymph node metastases (ie ≤3 vs >3, which is the cutoff for stage IIIa vs stage IIIb CRC in the Japanese Classification of Colorectal Cancer [Table 1]).16
Figure 2.
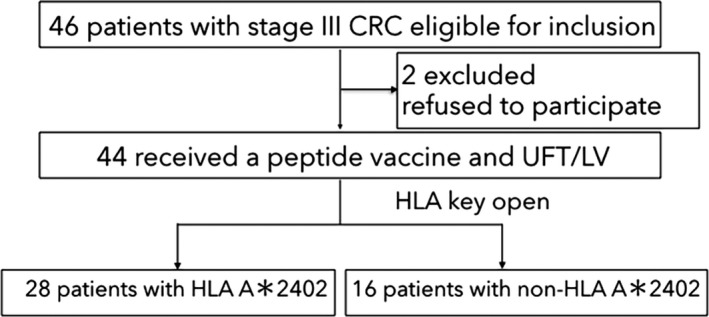
Flow of patients. CRC, colorectal cancer; UFT/LV, uracil‐tegafur/leucovorin
Table 1.
Patient background
| Total n = 44 | HLA A*2402 n = 28 | Non‐HLA A*2402 n = 16 | P | |
|---|---|---|---|---|
| Age (range) | 64 (37‐80) | 63 (37‐76) | 64 (47‐80) | n.s. |
| Gender, number (%) | ||||
| Male | 20 (45%) | 14 (50%) | 6 (37%) | n.s. |
| Female | 24 (55%) | 14 (50%) | 10 (63%) | |
| Number of vaccination, median (range) | 30 (21‐30) | 30 (21‐30) | 30 (30‐30) | n.s. |
| Colon/rectum, number (%) | ||||
| Colon | 25 (57%) | 17 (61%) | 7 (43%) | n.s. |
| Rectum | 19 (43%) | 11 (39%) | 9 (57%) | |
| Location of primary tumor, number (%) | ||||
| Right | 9 (20%) | 7 (25%) | 5 (31%) | n.s. |
| Left | 26 (60%) | 21 (75%) | 10 (69%) | |
| Stage, number (%) | ||||
| IIIa | 28 (64%) | 15 (54%) | 13 (81%) | n.s. |
| IIIb | 16 (36%) | 13 (46%) | 3 (19%) | |
n.s., not significant.
3.2. Immunological evaluation in the HLA‐A*2402‐matched and HLA‐A*2402‐unmatched groups
The median duration of follow‐up for the overall study population was 54 months (range: 11‐88 months). Peptide‐specific CTL responses were estimated by the in vitro ELISPOT assay before initiating therapy and after 2 cycles of treatment. A total of 35 patients could be assessed by ELISPOT assays after 2 cycles of treatment. In the HLA‐A*2402‐matched group, 14 patients had positive CTL responses specific for the RNF43 and/or TOMM34 peptides after 2 cycles of treatment, and 9 had negative responses; CTL responses of 5 patients were not evaluated (Table 2). In the HLA‐A*2402‐unmatched group, 10 patients had positive CTL responses specific for the RNF43 and/or TOMM34 peptides after 2 cycles of treatment and 2 had negative responses; CTL responses of 4 patients were not evaluated (Table 3).
Table 2.
In vitro enzyme‐linked immunospot assay before the initiation of therapy and after 2 cycles of treatment in the HLA‐A2402 matched group
| Number | CTL response (RNF43/TOMM34) | ||
|---|---|---|---|
| Before the initiation of therapy | After 2 cycles of therapy | Assessment | |
| 1 | +/+ | NA/NA | Not assessed |
| 2 | −/− | −/NA | Not assessed |
| 3 | −/+ | −/+ | Positive |
| 4 | −/− | +/− | Positive |
| 5 | −/+ | −/− | Negative |
| 6 | −/− | NA/NA | Not assessed |
| 7 | +/− | +/− | Positive |
| 8 | −/− | +/− | Positive |
| 9 | −/− | −/+ | Positive |
| 10 | −/− | −/− | Negative |
| 11 | −/− | +/− | Positive |
| 12 | −/− | −/− | Negative |
| 13 | +/− | +/− | Positive |
| 14 | +/− | −/+ | Positive |
| 15 | −/− | −/− | Negative |
| 16 | −/− | +/− | Positive |
| 17 | +/NA | +/+ | Positive |
| 18 | −/NA | −/− | Negative |
| 19 | −/− | −/− | Negative |
| 20 | −/− | −/− | Negative |
| 21 | −/− | −/− | Negative |
| 22 | +/+ | −/NA | Not assessed |
| 23 | −/− | +/NA | Positive |
| 24 | −/NA | −/− | Negative |
| 25 | −/− | −/+ | Positive |
| 26 | +/− | +/− | Positive |
| 27 | +/− | −/+ | Positive |
| 28 | −/− | −/NA | Not assessed |
NA, not available.
Table 3.
In vitro enzyme‐linked immunospot assay before the initiation of therapy and after two cycles of treatment in the HLA‐A2402 unmatched group
| No. | CTL Response (RNF43/TOMM34) | ||
|---|---|---|---|
| Before the initiation of therapy | After 2 cycles of therapy | Assessment | |
| 1 | NA/NA | NA/NA | Not assessed |
| 2 | −/− | +/+ | Positive |
| 3 | −/− | +/− | Positive |
| 4 | +/− | ++/+ | Positive |
| 5 | −/− | NA/− | Not assessed |
| 6 | −/− | +/+ | Positive |
| 7 | NA/NA | −/− | Negative |
| 8 | −/− | −/+ | Positive |
| 9 | +/− | −/+ | Positive |
| 10 | −/− | −/− | Negative |
| 11 | −/+ | +/+ | Positive |
| 12 | +/− | −/+ | Positive |
| 13 | −/− | −/+ | Positive |
| 14 | −/− | NA/NA | Not assessed |
| 15 | −/NA | +/− | Positive |
| 16 | −/NA | NA/NA | Not assessed |
NA, not available.
3.3. Correlation of CTL response after 2 cycles of treatment to survivals
Three‐year RFS was significantly better in patients with positive CTL response (87.3%) after 2 cycles of treatment than in those without (36.4%) in the total patients (HR = 0.16, 95% CI: 0.034‐0.58, P = .0050; Figure 3A). In the HLA‐A*2402‐matched group, 3‐year RFS was significantly better in patients with positive CTL responses (85.7%) after 2 cycles of treatment than in those without (33.3%) (HR = 0.16, 95% CI: 0.023‐0.70, P = .014; Figure 3B). In the HLA‐A*2402‐unmatched group, patients with positive CTL responses after 2 cycles of treatment had an equally good 3‐year RFS as did those in the HLA‐A*2402‐matched group (Figure 3C).
Figure 3.
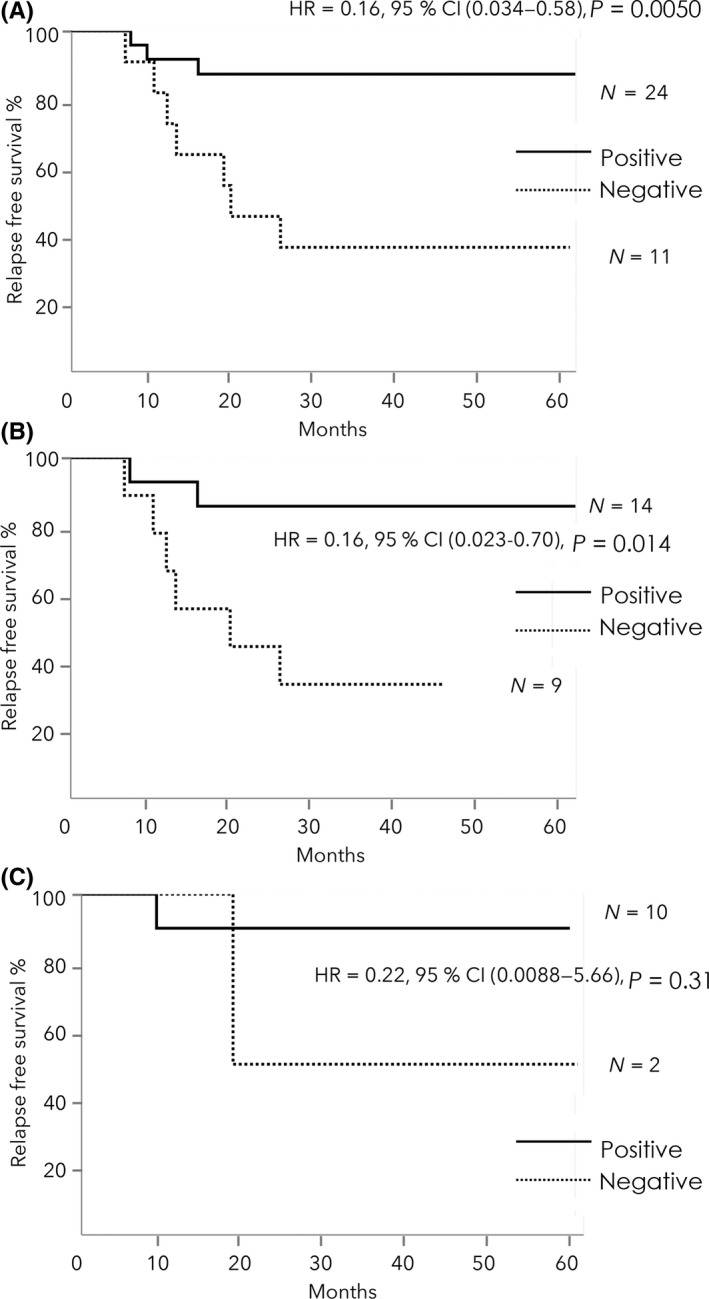
A, Kaplan‐Meier analysis of relapse‐free survival (RFS) in the total patients. The 3‐y RFS was significantly better in patients with positive CTL responses after 2 cycles of treatment than in those without, in the 2 groups. B, Kaplan‐Meier analysis of RFS in HLA‐A*2402‐matched group. The 3‐y RFS was significantly better in patients with positive CTL responses after 2 cycles of treatment than in those without. C, Kaplan‐Meier analysis of RFS in HLA‐A*2402‐unmatched group. RFS did not significantly differ between the positive and negative CTL response groups
3.4. Correlation of vaccination‐induced CTL response to survival
Out of 35 patients who could be assessed by ELISPOT assays after 2 cycles of treatment, 2 patients (No.17 in the HLA‐A*2402‐matched group and No.15 in the HLA‐A*2402‐unmatched group) patients were excluded in the analysis of vaccination‐induced CTL response due to missing data before the initiation of therapy. A total of 33 patients were assessed for vaccination‐induced CTL responses. The HLA‐A*2402‐matched group had 7 positive, 6 intermediate and 9 negative CTL responses to peptides after 2 cycles of treatment; and the HLA‐A*2402‐unmatched group had 7 positive, 2 intermediate and 2 negative CTL responses (Table 4).
Table 4.
Vaccination‐induced CTL responses before the therapy and after 2 cycles of treatment
| CTL responses (RNF43/TOMM34) | HLA‐A2402 | Assessment of vaccination‐induced CTL responses | ||
|---|---|---|---|---|
| Before the therapy | After 2 cycles of treatment | Matched group N = 22 | Unmatched group N = 11 | |
| −/− or −/+ or +/− | −/− | 9 | 2 | Negative |
| −/+ or +/− | −/+ or +/− | 4 | 0 | Intermediate |
| −/+ or +/− | +/− or −/+ | 2 | 2 | |
| −/− | −/+ or +/− or +/+ | 7 | 5 | Positive |
| −/+ or +/− | +/+ or ++/+ | 0 | 2 | |
Three‐year RFS was significantly better in patients with positive vaccination‐induced responses (92.9%) than in those with negative responses (36.4%) in the total patients (HR = 0.086, 95% CI: 0.0045‐0.49, P = .0035; Figure 4A). Three‐year RFS was also significantly better in patients with positive vaccination‐induced responses (85.7%) than in those with negative responses (33.3%) in the HLA‐A*2402‐matched group (HR = 0.16, 95% CI: 0.0087‐0.98, P = .047; Figure 4B). Patients with positive vaccination‐induced responses in the unmatched group also showed good RFS (Figure 4C).
Figure 4.
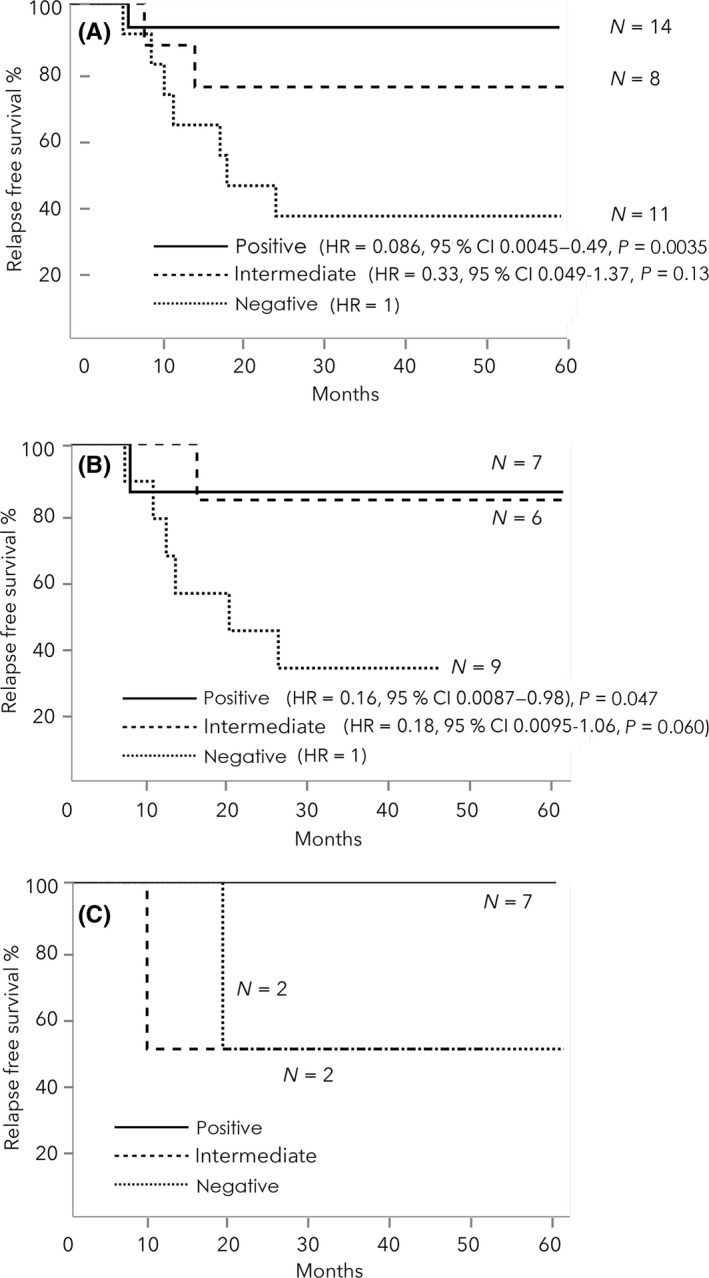
A, Kaplan‐Meier analysis of relapse‐free survival (RFS) in the total patients. The 3‐y RFS was significantly better in patients with positive vaccination‐induced responses than in those with negative responses. B, Kaplan‐Meier analysis of RFS in HLA‐A*2402 matched group. The 3‐y RFS was also significantly better in patients with positive vaccination‐induced responses than in those with negative responses. C, Kaplan‐Meier analysis of RFS in HLA‐A*2402‐unmatched group
4. DISCUSSION
In this study, both peptide‐specific CTL responses after 2 cycles of treatment and vaccination‐induced CTL responses were associated with better survival in patients with HLA‐A* 2402‐positive stage III CRC in adjuvant setting. In our earlier trial of the same peptides with UFT/LV, patients who showed immunological responses to both peptides had longer overall survival than did patients who showed responses to only 1 peptide or to none.8 Other reports have shown that immune response against multiple peptide antigens might improve survival for patients with advanced cancers.17, 18, 19 Our present study also supports the concept that vaccination‐induced immune response could improve prognosis of patients with advanced cancers. More importantly, our study showed that immune responses that occur shortly after initiating therapy could be used to predict the therapeutic effect.
Surprisingly, both peptide‐specific CTL responses and the vaccination‐induced CTL responses were also observed in HLA‐A24‐unmatched group; patients with positive CTL responses in this group had an equally good prognosis as in HLA‐A24‐matched group. Peptides used in this study were selected based on their predicted binding affinity to the HLA‐A*2402 molecule by BIMAS binding prediction software (Internet address: https://www-bimas.cit.nih.gov/molbio/hla_bind/), and are considered HLA‐A*2402‐restricted. The predicted binding affinities of the 9‐mer and 10‐mer peptides derived from RNF43 and TOMM34 to HLA‐A*24 with BIMAS software are higher than those to the other HLA serotypes (HLA‐A*1, A*2, A*3, A*11 and A*31) (Figure 5). Although the binding affinities of the peptides to the other HLA serotypes are not likely to be high, there might be a cross‐reaction of these peptides to other serotypes.
Figure 5.
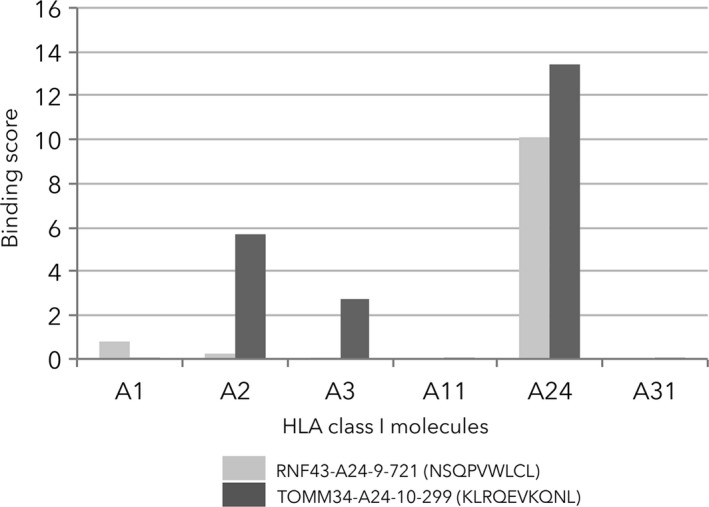
Predicted binding affinities of the 9‐mer and 10‐mer peptides derived from RNF43 and TOMM34 to HLA serotypes (HLA‐A*1, A*2, A*3, A*11, A*24 and A*31) with BIMAS software
This study was an HLA‐A status double‐blind, biologically randomized phase II study. As the peptides used are considered HLA‐A*2402‐restricted, the HLA‐A* 2402‐positive patient group was considered as the experimental arm and the HLA‐A* 2402‐negative group as the control arm. However, given the difficulties of predicting cross‐reactivity of the peptides to other serotypes (as described above), care must be taken in creating the study design.
The present study has some limitations. First, the sample size was small. Therefore, to confirm the survival benefit of peptide vaccination with UFT/LV for patients in HLA‐A24‐matched and HLA‐A24‐unmatched groups, additional patients should be recruited to achieve adequate statistical power, because some patients did not have measurable CTL responses specific for RNF43 and/or TOMM34 peptides. Second, this study did not compare outcomes between patients who received peptide vaccination and those who did not. An appropriate control would be HLA‐A* 2402‐positive CRC patients who received UFT/LV with no peptide vaccination, to assess the definite value of peptide vaccination.
In conclusion, vaccination‐induced immune response combined with UFT/LV could improve prognosis of HLA‐A* 2402‐positive patients with stage III CRC. Further study is needed to clarify whether vaccination‐induced immune response that occurs shortly after the initiating therapy can predict prognosis and enhance therapeutic strategies for stage III CRC.
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to declare.
ACKNOWLEDGMENTS
This study was supported partially by JSPS KAKENHI Grant Number 15K10153. We greatly appreciate the excellent advice and cooperation of Dr Sachiko Yoshimura from OncoTherapy Science and Professor Yusuke Nakamura at the University of Chicago, who also provided all the peptides used in this study. We also thank Marla Brunker from the Edanz Group (http://www.edanzediting.com) for editing a draft of this manuscript.
Kawamura J, Sugiura F, Sukegawa Y, et al. Cytotoxic T lymphocyte response to peptide vaccination predicts survival in stage III colorectal cancer. Cancer Sci. 2018;109:1545‐1551. https://doi.org/10.1111/cas.13547
REFERENCES
- 1. NIH consensus conference . Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444‐1450. [PubMed] [Google Scholar]
- 2. Andre T, Boni C, Mounedji‐Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. New Engl J Med. 2004;350:2343‐2351. [DOI] [PubMed] [Google Scholar]
- 3. Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C‐07. J Clin Oncol. 2007;25:2198‐2204. [DOI] [PubMed] [Google Scholar]
- 4. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. New Engl J Med. 2005;352:2696‐2704. [DOI] [PubMed] [Google Scholar]
- 5. Yoshida M, Ishiguro M, Ikejiri K, et al. S‐1 as adjuvant chemotherapy for stage III colon cancer: a randomized phase III study (ACTS‐CC trial). Ann Oncol. 2014;25:1743‐1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Onco. 2009;27:3109‐3116. [DOI] [PubMed] [Google Scholar]
- 7. Uchida N, Tsunoda T, Wada S, Furukawa Y, Nakamura Y, Tahara H. Ring finger protein 43 as a new target for cancer immunotherapy. Clin Cancer Res. 2004;10:8577‐8586. [DOI] [PubMed] [Google Scholar]
- 8. Okuno K, Sugiura F, Hida JI, et al. Phase I clinical trial of a novel peptide vaccine in combination with UFT/LV for metastatic colorectal cancer. Exp Ther Med. 2011;2:73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawamura J, Sugiura F, Sukegawa Y, et al. Multicenter, phase II clinical trial of peptide vaccination with oral chemotherapy following curative resection for stage III colorectal cancer. Oncol Lett. 2018;15:4241‐4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanaka F. UFT (tegafur and uracil) as postoperative adjuvant chemotherapy for solid tumors (carcinoma of the lung, stomach, colon/rectum, and breast): clinical evidence, mechanism of action, and future direction. Surg Today. 2007;37:923‐943. [DOI] [PubMed] [Google Scholar]
- 11. Carmichael J, Popiela T, Radstone D, et al. Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2002;20:3617‐3627. [DOI] [PubMed] [Google Scholar]
- 12. Douillard JY, Hoff PM, Skillings JR, et al. Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2002;20:3605‐3616. [DOI] [PubMed] [Google Scholar]
- 13. Shirao K, Hoff PM, Ohtsu A, et al. Comparison of the efficacy, toxicity, and pharmacokinetics of a uracil/tegafur (UFT) plus oral leucovorin (LV) regimen between Japanese and American patients with advanced colorectal cancer: Joint United States and Japan study of UFT/LV. J Clin Oncol. 2004;22:3466‐3474. [DOI] [PubMed] [Google Scholar]
- 14. Okuno K, Sugiura F, Inoue K, Sukegawa Y. Clinical trial of a 7‐peptide cocktail vaccine with oral chemotherapy for patients with metastatic colorectal cancer. Anticancer Res. 2014;34:3045‐3052. [PubMed] [Google Scholar]
- 15. Kono K, Iinuma H, Akutsu Y, et al. Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer‐testis antigens. J Trans Med. 2012;10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hazama S, Nakamura Y, Takenouchi H, et al. A phase I study of combination vaccine treatment of five therapeutic epitope‐peptides for metastatic colorectal cancer;safety, immunological response, and clinical outcome. J Trans Med. 2014;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single‐dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254‐1261. [DOI] [PubMed] [Google Scholar]
- 19. Obara W, Eto M, Mimata H, et al. A phase I/II study of cancer peptide vaccine S‐288310 in patients with advanced urothelial carcinoma of the bladder. Ann Oncol. 2017;28:798‐803. [DOI] [PubMed] [Google Scholar]


