Abstract
A previously healthy 58-year-old man was admitted for muscle pain and weakness [manual muscle testing (MMT) of 4/4 for upper and lower limbs]. We detected elevated levels of inflammatory makers and PR3-anti-neutrophil cytoplasmic antibody (ANCA). Subsequently, the muscle weakness rapidly progressed to an MMT of 2 for all limbs. Magnetic resonance imaging indicated muscle edema, and the creatine kinase (CK) level increased to 29,998 U/L. Methylprednisolone (mPSL) and cyclophosphamide pulse therapy improved the patient symptoms. MMT recovered to 4 for all limbs. A muscle biopsy showed degenerated muscle fibers surrounded by neutrophil-predominant infiltration. In addition, lamina elastic breakdown and fibrinoid necrosis of arterioles were observed. A final diagnosis of microscopic polyangiitis (MPA) limited to the muscles was made.
Keywords: ANCA-associated vasculitis,; organ limited vasculitis; PR3-ANCA
Introduction
Systemic vasculitis usually affects multiple organ systems. Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a common cause of systemic vasculitis (1). The disease frequently affects the lungs, kidneys, and nervous and cutaneous systems. In contrast to systemic vasculitis, vasculitis limited to single organs has also been identified (2). For example, renal- and pulmonary-limited types are considered mild forms of AAV (2, 3). In addition to these major target organs, other organs can occasionally be affected. For example, otitis media with AAV (OMAAV) is considered a part of limited organ-type vasculitis (4). Hepatic aneurysm is another a rare presentation of AAV (5-8), and retinal, breast, and urogenital structures can also become target organs on occasion (9). In addition, muscles can also become a target organ of vasculitis (10-12). Previous reports have shown that the lower limbs are primarily affected in vasculitis.
We herein report an unusual case of vasculitis that affects all limbs.
Case Report
A 58-year-old Japanese man was referred to our hospital for muscle pain and weakness. Two weeks before admission, the patient noticed left-knee pain. A prior physician diagnosed the patient as gout arthritis, given that the patient had a history of several previous gout attacks.
Non-steroidal anti-inflammatory drugs, vitamin B12, and allopurinol were administered. One week before admission, the patient experienced worsening pain in his limbs and was unable to stand up alone. The patient was then brought to the hospital. He had been healthy before this episode, except for having gout arthritis. He took no medications except for a prior prescription from his physician. He was a current smoker (40 packs over 40 years) and drank 1-2 L of beer daily. He had no history of traveling abroad or contact with animals during the last year.
The patient's height was 163 cm, and he weighed 61.9 kg. Involuntary weight loss was not reported. His blood pressure was 162/102 mmHg, heart rate was a regular 109 beats per minute, and body temperature was 37.5 °C. He reported not feeling cold. His respiratory rate was 16 breaths per minute. Percutaneous oxygen saturation (SpO2) was 97% in room air. A physical examination showed no remarkable findings expect for muscle weakness and tenderness. Manual muscle testing (MMT) was 4/5 for both his upper and lower limbs. The detailed MMT results were as follows: MMT (R/L) trapezius 4/4, deltoid 4/4, biceps 4/4, triceps 4/4, brachioradialis 4/4, iliopsoas 4/4, quadriceps 4/4, hamstrings 4/4, anterior tibialis 4/4, and gastrocnemius 4/4. The extent of muscle weakness was similar in the proximal and distal muscles. Grasping pain in the upper and lower limbs was also present. Neck flexor/extensor muscles were well preserved, and the patient could easily lift his neck. Dysphagia was not observed.
The patient's level of consciousness was clear. No paralysis or sensory disorder was observed. In addition, cranial nerve impairment and dysarthria were not detected. Stocking and glove distribution of sensory loss were also not observed. However, the patient complained of limb pain but did not complain of numbness, a burning sensation, or pain in his hands and feet. We could not identify swelling of his joints, muscles, or superficial lymph node. Swelling of his left knee was not obvious at admission.
No eruption or erythema were observed, nor was Gottron's sign detected. The results from the complete blood count, biochemical, coagulation, urinalysis, cerebrospinal fluid (CSF), and endocrinological tests are shown in Table 1. The white blood cell (WBC) count and C-reactive protein (CRP) levels were elevated. A slight abnormality in the liver function test was also found. The creatinine kinase (CK) level was normal at admission, and no electrolyte disorder was identified. Urinary testing did not suggest the presence of glomerulonephritis. A cerebrospinal fluid examination was also normal. In addition, the thyroid function and adrenal gland function were within normal ranges (Table 1); however, chest X-ray showed a mild emphysematous change. Consistent with this, computed tomography (CT) showed emphysematous changes and a solitary hepatic cyst. No interstitial pneumonia was detected. Electrocardiogram (ECG) testing showed normal sinus rhythm. Cardiac ultrasound showed good wall motion without vegetation or pericardial effusion. The patient was admitted for a further examination to focus on inflammation and the cause of his muscle weakness and tenderness.
Table 1.
Laboratory Data at Admission.
| <Complete Blood Count Data> | <Biochemistry Data> | <Biochemistry Data> | <CSF Analysis> | ||||||||
| WBC | 20,600 | /μL | TP | 6.3 | g/dL | IgG | 862 | mg/dL | Color | clear | |
| Neu | 90.5 | % | Alb | 2.9 | g/dL | IgA | 350 | mg/dL | Specific Gravity | 1.005 | |
| Lym | 4.5 | % | T-bil | 0.8 | mg/dL | IgM | 36 | mg/dL | Glu | 67 | mg/dL |
| Mono | 3.5 | % | AST | 50 | U/L | CH50 | 81.2 | CH50/mL | Protein | 17 | mg/dL |
| Eos | 1.0 | % | ALT | 55 | U/L | C3 | 183 | mg/dL | Cell | <1 | /μL |
| RBC | 474 | ×104/μL | LDH | 151 | U/L | C4 | 58 | mg/dL | LDH | 14 | IU/L |
| Hb | 14.4 | g/dL | γGTP | 119 | U/L | Vitamin B12 | >1,500 | pg/mL | CK | 24 | IU/L |
| Ht | 44.2 | % | T-chol | 166 | mg/dL | Vitamin B2 | 123.2 | ng/mL | <Endocrinological Data> | ||
| MCV | 93.1 | fl | TG | 157 | mg/dL | Vitamin B1 | 107 | ng/mL | ACTH | 5.9 | pg/mL |
| MCH | 30.3 | pg | BUN | 24.3 | mg/dL | Folate | 4.3 | ng/mL | Cortisol | 32.3 | μg/dL |
| MCHC | 32.6 | % | Cre | 0.88 | mg/dL | <Urinalysis> | TSH | 0.763 | μIU/mL | ||
| Plt | 58.8 | ×104/μL | Na | 130 | mEq/L | Specific Gravity | 1.017 | fT3 | 1.14 | ng/mL | |
| ESR | K | 5.3 | mEq/L | pH | 5.5 | fT4 | 1.45 | ng/dL | |||
| 1h | 63 | mm | Cl | 94 | mEq/L | UP | +/- | ||||
| 2h | 72 | mm | Ca | 8.7 | mg/dL | Glu | - | ||||
| <Coagulation> | P | 4.0 | mg/dL | uOB | - | ||||||
| PT-INR | 1.25 | UA | 5.2 | mg/dL | Ketone | 1+ | |||||
| APTT | 43.7 | sec | CK | 134 | U/L | WBC Elastase | - | ||||
| Fibrinogen | 929 | mg/dL | Glu | 111 | mg/dL | Nitrate | - | ||||
| HbA1c | 6.0 | % | <Urine Sedimentation> | ||||||||
| CRP | 30.5 | mg/dL | uRBC | 5-10 | /HPF | ||||||
| Ferritin | 816 | ng/mL | uWBC | 1-4 | /HPF | ||||||
| Aldolase | 6.6 | U/L | |||||||||
WBC: white blood cell count, RBC: red blood cell count, Hb: hemoglobin, Ht: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, Plt: platelet, ESR: erythrocyte sedimentation ratio, PT-INR: prothrombin time-international normalized ratio, APTT: activated partial thromboplastin tamest, total protein Alb: albumin, T-Bil: total bilirubin, D-Bil: direct bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, LDH: lactic dehydrogenase, γ-GTP: γ-glutamyl transpeptidase, T-Chol: total cholesterol, TG: triglyceride, BUN: blood urea nitrogen, Cr: creatinine, UA: uric acid, CK: creatine kinase, Glu: glucose, CRP: C-reactive protein, Ig: immunoglobulin, UP: proteinuria, uOB: urine occult blood, ACTH: adrenocorticotropic hormone, TSH: thyroid-stimulating hormone, fT3: free triiodothyronine, fT4: free thyroxine
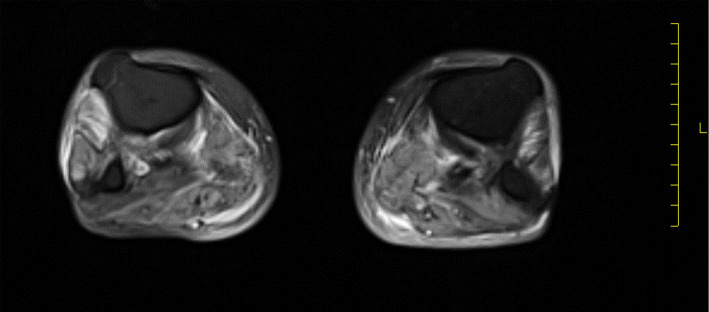
We initially suspected infection (including rickettsia) or alcoholic myopathy and initiated administration of ampicillin/sulbactam and minocycline. Two days after admission, the blood culture and CSF culture results were shown to be negative. Other cultures also failed to show bacterial growth. However, the patient's symptom did not change (Table 2). On day 5, the CK levels increased to 713 U/mL. Magnetic resonance imaging (MRI) of the lower limb showed diffuse high intensity on T2-weighted imaging (Fig. 1). On the 6th day after admission, his muscle weakness progressed, and the pain levels increased. On the 8th day, the patient's symptom continued to worsen. The serum CK levels increased to 19,430 U/mL, and MMT of the limbs decreased to 2/2. The MMT result details are as follows: MMT (R/L) trapezius 2/2, deltoid 2/2, biceps 4/4, triceps 2/2, brachioradialis 2/2, iliopsoas 2/2, quadriceps 2/2, hamstrings 2/2, anterior tibialis 2/2, and gastrocnemius 2/2. The degree of muscle weakness was similar in the proximal and distal muscles. The patient reported pain in the extremities as intense; however, no sensory disorders were detected. The consciousness level was clear. The neck flexor/extensor muscles were not affected, and dysarthria and dysphagia were not obvious. Cranial nerve disorder was also not apparent. The patient could describe his symptoms by himself. Dark urine was observed on day 8. Serum CK levels increased to 29,998 IU/mL, and serum creatinine levels increased to 1.34 mg/dL. We performed a muscle biopsy of the left gastrocnemius on day 8. On the same day, the proteinase 3 (PR3)-ANCA titer was found to be 65.8 U/mL. In contrast, the results for other auto-antibodies, including myeloperoxidase (MPO-ANCA), were negative (Table 2). We hypothesized that the cause of muscle weakness and pain was related to vasculitis, so methylprednisolone (mPSL) pulse therapy was started.
Table 2.
Laboratory Data of Infectious Disease Tests and Autoimmune Antibodies.
| <Infectious Disease Test> | <Autoimmune Antibody> | ||||
| PRP | Negative | ANA | <40 | ||
| TPHA | Negative | ds-DNA IgG | <10 | IU/mL | |
| HBsAg | Negative | Anti-RNP Ab | <7 | U/mL | |
| HCV | Negative | Anti-SS-A-Ab | <7 | U/mL | |
| ATLA | Negative | Anti-SS-B-Ab | <7 | U/mL | |
| HIV | Negative | Anti-Jo-1-Ab | <7 | U/mL | |
| Blood Culture | Negative | Anti-CCP-Ab | <0.6 | U/mL | |
| CSF Culture | Negative | Anti-Centromere-Ab | <5.0 | U/mL | |
| Urine Culture | Negative | PR3-ANCA | 65.8 | U/mL | |
| β-D-glucan | 7.1 | pg/mL | MPO-ANCA | <1.0 | U/mL |
| QTF | Negative | Anti-GBM-Ab | <2.0 | U/mL | |
| PCT | 0.61 | ng/mL | Anti-AchR-Ab | <0.2 | nmol/L |
| MMP-3 | 213 | ng/mL | |||
| C1q | <1.5 | μg/mL | |||
| Cryoglobulin | negative | ||||
| Anti-CLβ2GPI-Ab | 1.2 | U/mL | |||
| Anti-Cardiolipin-Ab | 8.0 | U/mL | |||
ANA: anti-nuclear antibodies, Ab: antibody, ds-DNA: double-stranded DNA, RNP: ribonucleoprotein, SS: Sjögren syndrome, CCP: cyclic citrullinated peptide, PR3: proteinase-3, ANCA: anti-neutrophil cytoplasmic antibody, MPO: myeloperoxidase, GBM: glomerular basement membrane, AchR: acetylcholine receptor, MMP: matrix metalloproteinase, CLβ2GPI: cardiolipin antibodyβ2-glycoprotein-1
Figure 1.
MRI of the lower limb. Diffuse edematous changes were identified on the bilateral leg. An increased T2 signal in the subcutaneous and deep fascia was not noticeable.
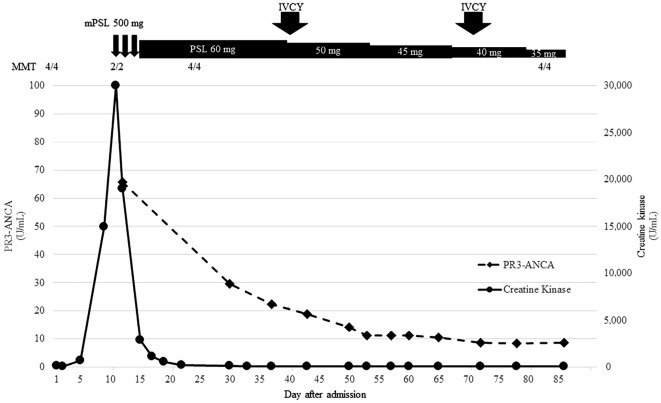
The patient's symptoms improved after treatment, and CK levels gradually decreased (Fig. 2). MMT recovered to 4/4. The detailed MMT results are as follows: MMT (R/L) trapezius 4/4, deltoid 4/4, biceps 4/4, triceps 4/4, brachioradialis 4/4, iliopsoas 4/4, quadriceps 4/4, hamstrings 4/4, anterior tibialis 4/4, and gastrocnemius 4/4. The extent of muscle weakness was similar in both the proximal and distal muscles.
Figure 2.
Clinical course. The y-axis on the left side shows the PR3-ANCA titer. The y-axis on the right side indicates the level of serum CK. CK was elevated on day 5 and peaked on day 11. The progression of muscle weakness and CK increase were highly correlated. The PR3-ANCA titer also decreased after treatment. After mPSL pulse therapy, CK gradually decreased to normal, and the muscle strength returned.
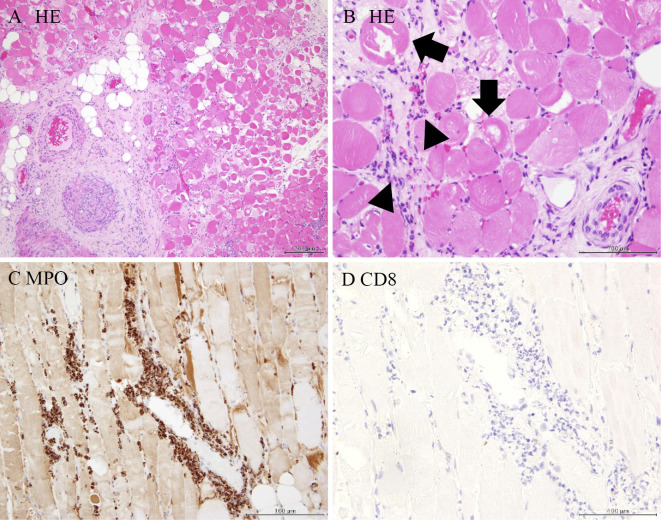
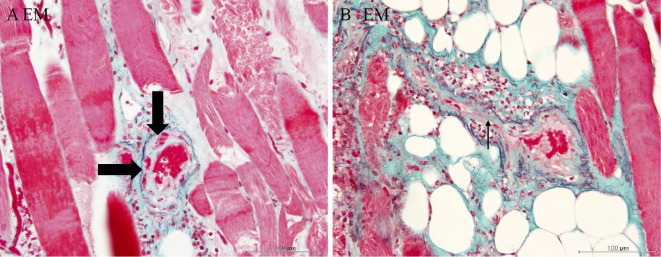
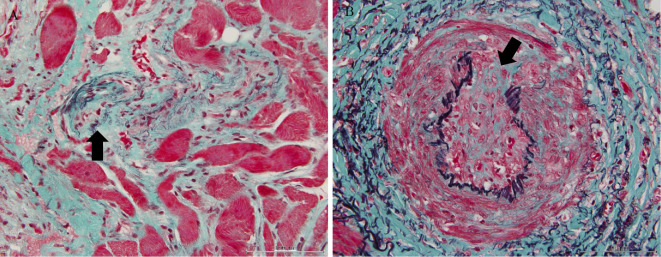
The muscle specimen showed severely degenerated muscle fiber surrounded by infiltrated neutrophils; few CD8-positive cells were detected (Fig. 3). Arterioles were narrowed due to intimal proliferation, and fibrinoid necrosis was identified (Fig. 4). Breakdown of the elastic lamina of the arteries was also observed (Fig. 5).
Figure 3.
A: Hematoxylin and Eosin (H&E) staining section of gastrocnemius revealed severely degenerated muscle fiber surrounded by infiltrated inflammatory cells, mainly neutrophils. (low-power field). B: H&E staining section of gastrocnemius. (high-power field). Degenerated muscle fiber (arrow) surrounded by infiltrated inflammatory cells (arrowhead). C: Myeloperoxidase (MPO) staining: MPO-positive cells (neutrophils) had infiltrated. D: CD8 staining. Few CD8-positive cells (lymphocyte) were observed.
Figure 4.
A: Elastica-Masson (EM) staining. Fibrinoid necrosis was found in the arteriole wall (arrow). B: An EM-stained section revealed small-artery stenosis by intimal proliferation (arrow).
Figure 5.
A: EM staining. Breakdown of the elastic lamina arteriole was detected (arrow). B: An EM-stained section revealed breakdown of the elastic lamina of a medium-sized artery (arrow).
We concluded that these findings were compatible with vasculitis. We gradually reduced the dose of oral prednisolone. To control vasculitis, cyclophosphamide pulse (10 mg/kg) therapy was administered on days 40 and 72. The patient was transferred to another hospital to continue rehabilitation on day 92. The patient underwent monthly intravenous cyclophosphamide (IVCY) 10 times and had an uneventful course without any complications.
Discussion
We report a case with an unusual presentation of vasculitis that affected all limbs. Immunosuppressive therapy was successful in controlling the disease.
We first thought that this patient was affected by alcoholic myopathy, infectious myopathy, or polymyositis/dermatomyositis (PM/DM). The patient had a history of substantial alcohol consumption, which was discontinued after hospitalization. Alcohol withdrawal symptoms were not observed. Previous reports suggest that the discontinuation of alcohol consumption improves symptoms (13-15). However, our case did not reveal any improvement after the cessation of alcohol intake. As such, the possibility of alcoholic myopathy was considered to be low.
We also considered infection-related myopathy. Many viral infections are known to cause myositis with rhabdomyolysis, such as influenza A, B, and H1N1 (subtype of influenza A virus) (16, 17); coxsackievirus (18); Epstein-Barr virus (19); herpes simplex virus (20); parainfluenza (21); adenovirus (22, 23); echovirus (24); cytomegalovirus (25); measles (26); varicella-zoster (27); human immunodeficiency virus (28, 29); and dengue (30). We could not asses the presence of antibodies against all of these diseases; however, HIV infection was indicated to be negative by serology. For viral infection, specific therapies seldom exist.
As an alternative diagnosis, we also considered scrub typhus (a mite-borne infectious disease caused by Orientia tsutsugamushi). Although the patient did not show signs of a rash or lymphadenopathy, he did have a fever and mild liver function abnormalities. We performed a thorough examination to find any evidence of bites, but none could be identified. We started minocycline empirically, but the patient's symptoms did not improve. Tsutugamusi IgM and IgG were not elevated. Although the patient did not show typical symptoms of pneumonia or acute pyelonephritis, we could not exclude the possibility of bacterial infection. After performing blood cultures and the QuantiFERON-3 G test, we empirically started ampicillin/sulbactam, but the patient's symptoms did not improve.
We also assessed the possibility of drug-induced vasculitis/rhabdomyolysis. Allopurinol was discontinued before admission, and we believe it was involved to a slight extent. Ampicillin/sulbactam and minocycline were started on the day of admission. These drugs may have caused rhabdomyolysis in this patient. Indeed, all drugs have the potential to cause rhabdomyolysis. We therefore cannot absolutely rule out the possibility of antibiotic-related rhabdomyolysis. However, the patient's symptoms gradually progressed before admission, and the patient was already affected by vasculitis upon admission. As one hypothesis, antibiotics might have exacerbated vasculitis in this case. In particular, minocycline can sometimes cause vasculitis (31). It is therefore possible that antibiotics might have exacerbated the patient's vasculitis.
Autoantibody titers did not show any increases, with the exception of PR3-ANCA. Increased ANCA titers are sometimes a false-positive result, especially if several organs are involved (32). For example, inflammatory bowel disease, infection, and malignancy occasionally result in an elevated ANCA titer (32). In Japan, AAV primarily targets the kidneys, lungs, and nervous system (33). There was a lack of convincing evidence that the patient's muscle pain and weakness were due to autoimmune disease. The patient's clinical symptoms satisfied some of the criteria for polymyositis, such as muscle weakness and pain, elevated serum CK levels, absence of arthritis, presence of systemic inflammation and muscle fiber degeneration, and inflammatory cell infiltration (34-36). In polymyositis, it is unusual for the Jo-1 antibody to be negative and PR3-ANCA levels to be elevated.
In the present case, muscle weakness progressed rapidly, and we were unable to wait until a perfect diagnosis could be made. We therefore started the patient on immunosuppressant therapy, which proved highly effective.
We examined the validity of our diagnosis of vasculitis. Systemic vasculitis sometimes involves peripheral neuropathy (37-39). Suppiah et al. reported that the incidence of vasculitis motor-involving neuropathy was between 7% of microscopic polyangiitis (MPA) and 7% of granulomatosis with polyangiitis (GPA). About 50-75% of cases with polyarteritis nodosa (PN) involve the peripheral nervous system (38). These studies examined peripheral neuropathy as a systemic symptom (39). However, nephropathy was not accompanied by elevated CK levels. We were able to confirm muscular degeneration by MRI and a biopsy in this case. We concluded that the muscle was the main cause of the weakness.
Several studies have reported vasculitis limited to the limbs. Miyashita et al. reported 7 cases of lower limb-restricted vasculitis (10), and Khellaf et al. reported 11 cases of lower limb-restricted vasculitis (11). In these reports, the upper limbs were not involved. Benz et al. also reported three cases of muscle-limited vasculitis (12). These studies support our diagnosis of limb-limited vasculitis. Previous reports were unable to distinguish PN from AAV. We next considered the type of vasculitis in our case. According to the Watts criteria for the classification of vasculitis, our case was classified as MPA (40). Our case was not accompanied by eosinophilia or granuloma in the biopsy specimens. A muscle biopsy showed inflammation of both the arterioles and medium-sized arteries and was positive for PR3-ANCA. These data are more closely compatible with MPA than PN.
Alternatively, this may be a case of polymyositis (PM). As mentioned above, the clinical presentation mostly satisfied the diagnostic criteria of PM. However, some characteristics were different from those of PM. PM is thought to primarily cause muscle weakness in the proximal muscles. Instead, our case showed similar muscle weakness in both the proximal and distal muscles. Thus, on this clinical point, this case differed from that expected of PM. Furthermore, regarding the pathology of our case, several results differed from those expected if the patient had PM. For example, PM muscle pathology is characterized by cellular infiltrate (predominantly macrophage and lymphocyte) in the muscle fiber and no signs of vasculopathy (41, 42). In contrast, as shown in Fig. 3, we found that inflammatory cell infiltration was localized around the muscle fiber, and minimal cell infiltration was observed in the muscle fiber. Neutrophils were the predominant cell type present in the specimens (Fig. 3), and arteriole occlusion was observed (Fig. 4, 5). These findings support the diagnosis of vasculitis and the involvement of arterioles.
In contrast, PN usually affects medium-sized vessels (diameter 100-150 μm), and this finding was also observed in our case. However, according to the Watts algorithm, MPA was classified before PN. Taking these findings together, we conclude that this patient was affected by MPA. However, the coexistence of MPA and PN cannot be ruled out.
Another differential diagnosis that needed to be excluded was neutrophilic myositis, which sometimes accompanies ulcerative colitis and celiac disease (43, 44). However, our case did not show colitis or other celiac disease, and the disease background differed from those in previous reports (43, 44). Previous reports of neutrophilic myositis did not show accompanying vasculitis. The question we addressed was where was the main location of inflammation in this case-the vessel or the muscle itself. We understand that it is impossible to perfectly classify the location of inflammation. Prayton reported that necrotizing myositis was mainly caused by vessels, whereas degenerate and/or regenerate muscle fibers were mainly caused by neurogenic disorders (45). One suggestive case report by Parent et al. described a case of eosinophilic granulomatosis with polyangiitis (EGPA) presenting as diffuse myositis. In that report, a muscle biopsy showed necrotizing vasculitis with true myositis (46), and the main location of inflammation was thought to be both the vessels that supply the muscles and the muscle itself. Our case shows that both perimysial and endomysial infiltration of neutrophils can accompany vasculitis (Fig. 3A, B). The progression of the disease was rapid (Fig. 2). Inflammation of the vessels gradually narrowed the arteries, which became occluded. This interpretation seems to match the pathological mechanism identified in our case. Perimysial and endomysial infiltration of neutrophils may be a result of infarction or the spread of vasculitis. Taking these finding together, multiple small infarctions of the artery supplying skeletal muscles were the main cause in our case. The further examination of similar diseases is necessary to elucidate the pathological mechanism involved.
Previous reports have shown that immunosuppressive treatment (steroid and/or cyclophosphamide) was successful in controlling the disease (10-12). Since vasculitis limited to the muscle is extremely rare, we were unable to anticipate the prognosis of this case. Careful observation for relapse of the disease is needed.
In conclusion, we herein reported a case of MPA limited to muscle symptoms (pain and weakness). Our patient responded well to steroid and cyclophosphamide therapy. In the Ishinomaki region, the incidence of MPA increased after the Great East Japan earthquake (47). As the incidence of MPA increases, the number of cases with variable MPA phenotypes will also increase. This unusual case may help minimize the possibility of a missed diagnosis of AAV.
Informed consent was obtained from the patient. All procedures were carried out in accordance with the Declaration of Helsinki.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We greatly appreciate the valuable assistance of Yuko Ise for preparing additional immunohistochemistry specimens.
References
- 1. Jennette JC, Falk RJ, Bacon PA, et al. . 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1-11, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Ozaki S. Clinical trial for Japanese patients with myeloperoxidase anti-neutrophil cytoplasmic antibody-associated vasculitis: The JMAAV study. Clin Exp Nephrol 17: 700-704, 2013. [DOI] [PubMed] [Google Scholar]
- 3. Ozaki S, Atsumi T, Hayashi T, et al. . Severity-based treatment for Japanese patients with MPO-ANCA-associated vasculitis: The JMAAV study. Mod Rheumatol 22: 394-404, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harabuchi Y, Kishibe K, Tateyama K, et al. . Clinical features and treatment outcomes of otitis media with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (OMAAV): A retrospective analysis of 235 patients from a nationwide survey in Japan. Mod Rheumatol 27: 87-94, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Sato H, Matsuda K, Takeuchi Y, Fukami H, Saito A, Nagasawa T. [Case report; a case of vasculitis with multiple hepatic artery aneurysms]. Nihon Naika Gakkai Zasshi (Journal of Japanese Society of Internal Medicine) 105: 87-90, 2016. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 6. Teraoka H, Takeuchi K, Sakurai K, Nitta A. [A case of polyarteritis nodosa with intra-abdominal bleeding due to rupture of the hepatic aneurysm]. Nihon Shoukaki Gakkai Zasshi (Journal of Japanese Society of Internal Medicine) 103: 650-654, 2016. (in Japanese). [PubMed] [Google Scholar]
- 7. Kanai R, Nakamura M, Tomisato K, et al. . Cholangitis as an initial manifestation of polyarteritis nodosa. Intern Med 53: 2307-2312, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Nakamura S, Yokoi Y, Suzuki S, et al. . A case of melena caused by a hepatic aneurysm ruptured into the intrahepatic bile duct in a patient with allergic granulomatous angiitis. Jpn J Sur 21: 471-475, 1991. [DOI] [PubMed] [Google Scholar]
- 9. Atisha-Fregoso Y, Hinojosa-Azaola A, Alcocer-Varela J. Localized, single-organ vasculitis: clinical presentation and management. Clin Rheumatol 32: 1-6, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Miyashita N, Ayano M, Nakazawa T, Yokota T. Fever of unknown origin caused by vasculitis restricted to the lower limbs. Annual Report of Kurashiki Central Hospital: 263-268, 2010. (in Japanese). [Google Scholar]
- 11. Khellaf M, Hamidou M, Pagnoux C, et al. . Vasculitis restricted to the lower limbs: a clinical and histopathological study. Ann Rheum Dis 66: 554-556, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benz N, Daikeler T, Frank S, Mehling M, Tyndall A, Trendelenburg M. Three cases of primary small vessel vasculitis of the skeletal muscle-an own entity. BMJ Case Rep 2011: 2-5, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin F, Ward K, Slavin G, Levi J, Peters TJ. Alcoholic skeletal myopathy, a clinical and pathological study. Q J Med 55: 233-251, 1985. [PubMed] [Google Scholar]
- 14. Bleich HL, Moore MJ, Rubin E. Alcoholic myopathy in heart and skeletal muscle. N Engl J Med 301: 28-33, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Preedy VR, Adachi J, Peters TJ, et al. . Recent advances in the pathology of alcoholic myopathy. Alcohol Clin Exp Res 25: 54S-59S, 2001. [DOI] [PubMed] [Google Scholar]
- 16. Abe M, Higuchi T, Okada K, Kaizu K, Matsumoto K. Clinical study of influenza-associated rhabdomyolysis with acute renal failure. Clin Nephrol 66: 166-170, 2006. [DOI] [PubMed] [Google Scholar]
- 17. D'Silva D, Hewagama S, Doherty R, Korman TM, Buttery J. Melting muscles: novel H1N1 influenza A associated rhabdomyolysis. Pediatr Infect Dis J 28: 1138-1139, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Fodili F, van Bommel EF. Severe rhabdomyolysis and acute renal failure following recent Coxsackie B virus infection. Neth J Med 61: 177, 2003. [PubMed] [Google Scholar]
- 19. Friedman BI, Libby R. Epstein-Barr virus infection associated with rhabdomyolysis and acute renal failure. Clin Pediatr (Phila) 25: 228-229, 1986. [DOI] [PubMed] [Google Scholar]
- 20. Schlesinger JJ, Gandara D, Bensch KG. Myoglobinuria associated with herpes-group viral infections. Arch Intern Med 138: 422-424, 1978. [PubMed] [Google Scholar]
- 21. O'Connor JV, Iyer SK. Myoglobinuria associated with parainfluenza type 2 infection. NY State J Med 82: 1469-1470, 1982. [PubMed] [Google Scholar]
- 22. Wright J, Couchonnal G, Hodges GR. Adenovirus type 21 infection. Occurrence with pneumonia, rhabdomyolysis, and myoglobinuria in an adult. JAMA 241: 2420-2421, 1979. [DOI] [PubMed] [Google Scholar]
- 23. Meshkinpour H, Vaziri ND. Association of myoglobinuria with adenovirus infection. West J Med 137: 130-132, 1982. [PMC free article] [PubMed] [Google Scholar]
- 24. Josselson J, Pula T, Sadler JH. Acute rhabdomyolysis associated with an echovirus 9 infection. Arch Intern Med 140: 1671-1672, 1980. [PubMed] [Google Scholar]
- 25. Hughes GS, Hunt R. Cytomegalovirus infection with rhabdomyolysis and myoglobinuria. Ann Intern Med 101: 276-277, 1984. [DOI] [PubMed] [Google Scholar]
- 26. Seibold S, Merkel F, Weber M, Marx M. Rhabdomyolysis and acute renal failure in an adult with measles virus infection. Nephrol Dial Transplant 13: 1829-1831, 1998. [DOI] [PubMed] [Google Scholar]
- 27. Hollenstein U, Thalhammer F, Burgmann H. Disseminated intravascular coagulation (DIC) and rhabdomyolysis in fulminant varicella infection-case report and review of the literature. Infection 26: 306-308, 1998. [DOI] [PubMed] [Google Scholar]
- 28. Mahé A, Bruet A, Chabin E, Fendler JP. Acute rhabdomyolysis coincident with primary HIV-1 infection. Lancet 2: 1454-1455, 1989. [DOI] [PubMed] [Google Scholar]
- 29. Guillaume MP, Van Beers D, Delforge ML, Devriendt J, Cogan E. Primary human immunodeficiency virus infection presenting as myopericarditis and rhabdomyolysis. Clin Infect Dis 21: 451-452, 1995. [DOI] [PubMed] [Google Scholar]
- 30. Acharya S, Shukla S, Mahajan SN, Diwan SK. Acute dengue myositis with rhabdomyolysis and acute renal failure. Ann Indian Acad Neurol 13: 221-222, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lenert P, Icardi M, Dahmoush L. ANA (+) ANCA (+) systemic vasculitis associated with the use of minocycline: case-based review. Clin Rheumatol 32: 1099-1106, 2013. [DOI] [PubMed] [Google Scholar]
- 32. Houben E, Bax WA, van Dam B, et al. . Diagnosing ANCA-associated vasculitis in ANCA positive patients. Medicine (Baltimore) 95: e5096, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iwabuchi M, Nakaya I, Tsuchiya Y, et al. . Effects of cyclophosphamide on the prognosis of Japanese patients with renal vasculitis associated with anti-neutrophil cytoplasmic antibody-positive microscopic polyangiitis. Clin Exp Nephrol 20: 712-719, 2016. [DOI] [PubMed] [Google Scholar]
- 34. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292: 344-347, 1975. [DOI] [PubMed] [Google Scholar]
- 35. Targoff IN, Miller FW, Medsger TA, Oddis CV. Classification criteria for the idiopathic inflammatory myopathies. Curr Opin Rheumatol 9: 527-535, 1997. [DOI] [PubMed] [Google Scholar]
- 36. Tanimoto K, Nakano K, Kano S, et al. . Classification criteria for polymyositis and dermatomyositis. J Rheumatol 22: 668-674, 1995. [PubMed] [Google Scholar]
- 37. Mathew L, Talbot K, Love S, Puvanarajah S, Donaghy M. Treatment of vasculitic peripheral neuropathy: a retrospective analysis of outcome. QJM 100: 41-51, 2007. [DOI] [PubMed] [Google Scholar]
- 38. Pagnoux C, Guillevin L. Peripheral neuropathy in systemic vasculitides. Curr Opin Rheumatol 17: 41-48, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Suppiah R, Hadden RDM, Batra R, et al. . Peripheral neuropathy in ANCA-associated vasculitis: outcomes from the European Vasculitis Study Group trials. Rheumatology (Oxford) 50: 2214-2222, 2011. [DOI] [PubMed] [Google Scholar]
- 40. Watts R, Lane S, Hanslik T, et al. . Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 66: 222-227, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hohlfeld R, Engel AG. The immunobiology of muscle. Immunol Today 15: 269-274, 1994. [DOI] [PubMed] [Google Scholar]
- 42. Amato AA, Barohn RJ. Evaluation and treatment of inflammatory myopathies. J Neurol Neurosurg Psychiatry 80: 1060-1068, 2009. [DOI] [PubMed] [Google Scholar]
- 43. Qureshi JA, Staugaitis SM, Calabrese LH. Neutrophilic myositis: An extra-intestinal manifestation of ulcerative colitis. J Clin Rheum 8: 85-88, 2002. [DOI] [PubMed] [Google Scholar]
- 44. Alawneh K, Ashley C, Carlson JA. Neutrophilic myositis as a manifestation of celiac disease: a case report. Clin Rheumatol 27: S11-S13, 2008. [DOI] [PubMed] [Google Scholar]
- 45. Prayson RA. Skeletal muscle vasculitis exclusive of inflammatory myopathic conditions: a clinicopathologic study of 40 patients. Hum Pathol 33: 989-995, 2002. [DOI] [PubMed] [Google Scholar]
- 46. Parent ME, Larue S, Ellezam B. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) presenting as diffuse myositis. BMC Musculoskelet Disord 15: 388, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takeuchi Y, Saito A, Ojima Y, et al. . The influence of the Great East Japan earthquake on microscopic polyangiitis: a retrospective observational study. PLoS One 12: e0177482, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]