Abstract
Introduction:
Considering the increasing trend in incidence rates, morbidity and mortality of breast cancer, there is an urgent need to identify and validate new biomarkers for early detection and better management. The purpose of the study was to investigate BRCA1 protein expression and promoter methylation of the BRCA1 gene and their association with molecular subtypes based on estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) positivity.
Materials and Methods:
A total of 114 breast cancer tissue biopsies were collected for methylation specific PCR (MSP) and immunohistochemical (IHC) analysis.
Results:
Seven tissue microarrays were constructed. BRCA1 protein expression was reduced in 55/114 (48.2%) and in the majority of ER-negative tumors (73.3%) (p<0.001). Similarly BRCA1 expression was reduced in the majority of PR-negative tumors (69.2%) but without statistical significance (p value=0.083). BRCA1 methylation was positive in 59.6% cases. A subset regarding ER+, PR+ and HER2+ was identified which consisted of 31.6% in which an inverse relationship between BRCA1 methylation and protein expression was noted.
Conclusion:
Reduced expression was associated with ER and PR negative status which is linked with a poor prognosis. BRCA1 protein expression might thus be used as a prognostic indicator to predict treatment response to hormone therapy.
Keywords: Breast cancer, BRCA1, ER, PR, HER2 Neu
Introduction
Breast cancer is most commonly diagnosed cancer and leading cause of cancer death in female worldwide (Jamal et al., 2011). With urbanization and changes in lifestyle, there is increasing the incidence of carcinoma of the breast and it is estimated that every year 1,44,937 women are diagnosed with breast cancer and approximately 70,218 deaths due to this cancer (IARC, WHO, 2012). The incidence of breast cancer is higher in urban India, especially the metropolitan cities where it is now the leading female cancer and is the second most common cancer after cervix (IARC, WHO, 2012).
The most important biomarker in breast cancer is estrogen receptor (ER) expression as it gives information about the sensitivity of breast cancer to tamoxifen. Several studies have shown that ER-negative cases achieve pathological complete response with neoadjuvant chemotherapy compared with ER-positive cases (Weigel and Dowsett, 2010). Elledge et al., (2000) have shown that in metastatic breast cancer, the tumors observed ER and progesterone receptor (PR) positivity showed a better response to tamoxifen than tumors showing ER positivity but lacking (PR) expression. Human epidermal growth factor receptor 2 (HER2) positive (over-expressed) cases are seen to have more chances of relapse and shorter survival and indicator of poorer response to tamoxifen. However, with the advent of anti-HER2 therapies like monoclonal antibody Transtuzumab targeted at HER2, it needs to be incorporated in the testing panel as patients are highly benefited. Patients expressing truncated cytoplasmic HER2 are poorly responsive to trastuzumab but may be responsive to tyrosine kinase inhibitor lapatinib (Weigel and Dowsett, 2010).
Among the genes that have been associated with the genesis of breast cancer, the abnormalities of BRCA1 and BRCA2 genes are strongly implicated in the pathogenesis; both genes have been now cloned and fully characterized (Miki et al., 1994; Wooster et al., 1995). BRCA1 is a putative tumor suppressor gene located on chromosome 17q21 and its encodes a protein of 220 kDa consisting of 1863 amino acids. The BRCA1 protein is localized exclusively in the nuclei of normal and malignant breast tissue. Several investigators Dinesh et al., (2006) and Tulchin et al., (2013) have reported that BRCA1 protein expression is reduced or absent in familial and sporadic breast cancer by Immunohistochemical analysis. Mechanisms other than direct mutation of BRCA1 gene, such as allelic loss or methylations of the a BRCA1 promoter region (Miyamoto et al., 2002) may be involved in its altered protein expression. Our data also suggest that reduced expression of BRCA1 protein may play an important role in mammary carcinogenesis in Indian sporadic cases. Therefore, the present study has been designed to analyze BRCA1 promoter methylation and protein expression in breast cancer cases and correlate the expression of these proteins with various clinico-pathological parameters, ER, PR and HER2.
Materials and Methods
A total of 114 breast cancer cases were collected from Department of Surgery at University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi. Patients who had a cytological / histo-pathological diagnosis of breast malignancy were included in this study. Corresponding normal adjacent breast tissues of these patients were used as a control. The histological typing and Bloom Richardson’s grading was done by a trained pathologist. Ethical clearance obtains from the college ethical committee.
DNA extraction
High molecular weight genomic DNA from breast cancer biopsies and normal controls were isolated by standard Proteinase K digestion and phenol-chloroform extraction procedure described by Hedau et al., (2011).
Bisulfite modification
DNA methylation was carried out by sodium bisulfite treatment of genomic DNA. Briefly, 2 µg of genomic DNA was denatured with 0.3M NaOH for 15 min at 37 °C in a final volume of 20 µl followed by manufactures instruction (WizardR DNA clean-up Resin, Promega, USA).
Analysis of BRCA1 promoter methylation patterns
Methylation –specific PCR distinguishes unmethylated from methylated alleles in a given gene on the basis of sequence changes produced following bisulfite treatment of DNA, which convert unmethylated, but no methylated cytosine to uracil and subsequent PCR by use of primers designed for either methylated or unmethylated DNA (Sakakibara et al., 1996). Primer sequences of BRCA1 for the unmethylated reaction were 5’ – TTG GTT TTT GTG GTA ATG GAA AAG TGT – 3’ (sense) and 5’ – CAA AAA ATC TCA ACA AAC TCA CAC CA – 3’ (antisense) and for the methylated reaction 5’ – TCG TGG TAA CGC AAA AGC GC – 3’ (sense) and 5’ – AAA TCT CAA CGA ACT CAC GCC G – 3’ (antisense). The unmethylated product is 86bp long and the methylated product is 75bp. This region crosses the major transcription start size at 1581bp (Xu et al., 1995). Placental DNA treated in vitro with SssI bacterial methylase was used as a positive control for methylated genes. Ten microliters of each PCR product was loaded directly onto non-denaturing 6% polyacrylamide gels, stained with ethidium bromide and visualized under UV transilluminator.
Immunohistochemistry of BRCA1, ER, PR and HER2
An Immunohistochemical demonstration of BRCA1, ER, PR and HER2, 3-4 µm thick sections were deparaffinized with xylene, rehydrated through a graded alcohol series and brought to water. according to manufactures instructions. The sections were incubated with the monoclonal anti BRCA1 protein antibody (Mouse monoclonal anti BRCA1, clone MS110, USA) anti-ER protein antibody (Rabbit monoclonal anti ER, clone SP1, UK), anti- PR (Rabbit monoclonal anti PR, clone SP2 ,UK) and anti HER2 (Mouse monoclonal anti-HER2, clone CB-11, USA) followed by manufactures instructions.
Statistical Analysis
The association between BRCA1 protein expression, BRCA1 methylation, ER, PR, HER2 and clinico-pathological parameters Chi-square/Fisher’s exact test was used. Statistical analysis was done using statistical software SPSS version 20.
Results
A total of 114 cases were included in the study and all were histological/cytological confirmed cases of breast carcinoma. The age, type of the tumor, T stage, grade, lymph node status, presence or absence of an in-situ component, hormonal receptors (ER/PR) status, HER2 status were recorded in each case. The BRCA1, ER, PR and the HER2 expression were detected by immunohistochemistry (IHC) on tissue microarray sections. The age of the patients included in the study ranged from 24 to 80 years with the average age 45.1 years with a standard deviation of 10.95 years.
Comparisons between BRCA1 protein expression and different clinicopathological parameters
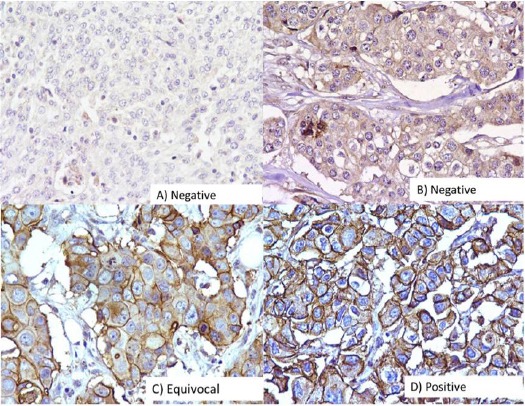
BRCA1 protein expression was studied in 114 breast cancer cases, out of which 55/114 (48.2%) cases showed reduced or absent expression while 42/114 (36.8%) cases showed moderate protein expression and 17/114 (15.0%) strong protein expression as compared to control (Figure 1).
Figure 1.
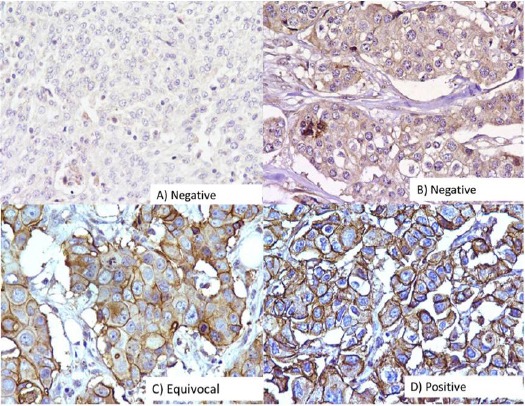
Immunohistochemical Analysis of BRCA1 in Breast Carcinomas. The photographs show (a) negative control showing no detectable BRCA1 immunoreactivity in which BRCA1 antibody has been replaced with isotype specific IgG with score 0 (b) weak expression with score 1-4 (c) moderate expression with score 5-8 and (d) strong expression with score 9-12; (A-D, original magnification ×200).
Among 114 cases, 48 (42.1%) were premenopausal and 66 (57.9%) were postmenopausal women. Out of 48 premenopausal cases, 25/48 (52%) cases had reduced BRCA1 expression, 11/48 (23%) moderate and 12/48 (25%) strong expression. While in 66 postmenopausal cases, 29/66 (43.9%) cases had reduced BRCA1 expression, 14/66 (21.3%) moderate and 23/66 (34.8%) strong expression, however, the difference was not statistically significant (p<0.52).
Estrogen receptors were studied in 114 cases, out of which 77 (67.50%) were ER-positive and 37 (32.50%) ER-negative (Figure 2). The statistical association was seen among BRCA1 expression reduced/moderate to strong expression and ER status (p <0.001). ER-positive tumors were significantly seen to be associated with lower tumor size (p <0.026) (Table 1).
Figure 2.
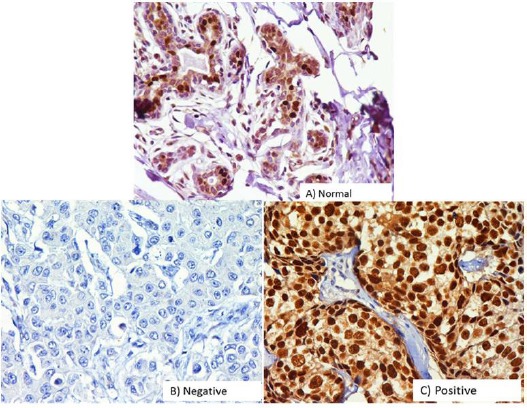
Immunohistochemical Analysis of Estrogens Receptor (ER) in Breast Carcinomas. The photographs show (a) normal breast tissue (b) negative control showing no detectable ER immunoreactivity in which ER antibody has been replaced with isotype specific IgG. (c) Positive expression (A-D, original magnification ×200).
Table 1.
Comparison of BRCA1 Expression and Age, Menopausal Status, ER, PR and HER2 Status (n=114)
| Age | BRCA1 Expression | p value | |||
|---|---|---|---|---|---|
| Below 50 years | 74 (64.9%) | ||||
| Above 50 years | 40 (35.1%) | ||||
| Menopausal status | Reduced | Moderate | Strong | (p>0.5210) | |
| Pre-menopausal 48 (42.1%) | 25 (52%) | 11 (23%) | 12 (25%) | ||
| Post-menopausal 66 (57.9%) | 29 (43.9%) | 14 (21.3%) | 23 (24.8%) | ||
| ER Expression | Absent (15) | Weak (49) | Moderate (21) | Strong (29) | (p<0.0005) |
| ER Positive 77 (67.5%) | 4 (26.7%) | 28 (57.1) | 16 (76.2%) | 25 (86.2%) | |
| ER Negative 37 (32.5%) | 11(73.3%) | 21 (42.9) | 5 (23.8%) | 4 (13.8%) | |
| PR Expression | Absent (13) | Weak (51) | Moderate (23) | Strong (27) | (p>0.833) |
| PR Positive 48 (42.1%) | 4 (30.8%) | 16 (31.4%) | 11 (47.8%) | 16 (59.3%) | |
| PR Negative 66 (57.9%) | 9 (69.2%) | 35 (68.6%) | 12 (52.2%) | 11 (40.7%) | |
| HER 2 Expression | Absent (17) | Weak (45) | Moderate (25) | Strong (27) | (p>0.1294) |
| HER Positive 43 (37.8%) | 3 (17.7%) | 15 (33.3%) | 12 (48.0%) | 13 (48.2%) | |
| HER 2 Negative 71 (62.2%) | 14 (82.3%) | 30 (66.7%) | 13 (52.0%) | 14 (51.8%) |
Progesterone receptor was studied in 114 cases out of which 48 (42.1%) were PR-positive and 66 (57.9%) were PR-negative (Figure 3). No statistical association was seen among BRCA1 reduced/moderate to strong protein expression and PR expression (p <0.083). HER2 expression was analyzed in 114 cases out of which 43 (37.8%) were HER2 positive and 71(62.2%) were HER2 negative (Figure 4). No statistically significant association was seen among BRCA1 reduced/moderate to strong protein expression and HER2 expression (p<0.129).
Figure 3.
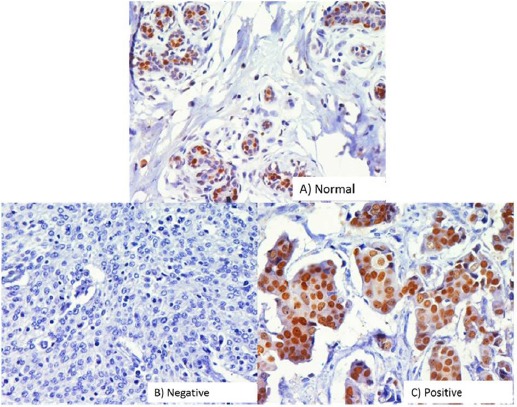
Immunohistochemical Analysis of Progesterone Receptor (PR) in Breast Carcinomas. The photographs show (a) normal breast tissue (b) negative control showing no detectable PR immunoreactivity in which PR antibody has been replaced with isotype specific IgG. (c) Positive expression (A-D, original magnification ×200).
Figure 4.
Immunohistochemical Analysis of HER2 in Breast Carcinomas. The photographs show (a) negative control showing no detectable HER2 immunoreactivity in which HER2 antibody has been replaced with isotype specific IgG. (b) Equivocal and (c) Positive expression (A-D, original magnification ×200).
Similarly, no statistical association was observed between, histological subtypes, grade of the tumor, tumor staging, in-situ component, lymph node status, radiation therapy/cytotoxic therapy (RT/CT) status and BRCA1 protein expression.
Comparisons between BRCA1 promoter methylation and different clinicopathological parameters
BRCA1 promoter methylation was studied in 114 breast cancer tissues, out of which 68/114 (59.6%) cases showed promoter methylation while 46/114 (40.4%) cases showed the absence of methylation or no methylation (Figure 5). BRCA1 promoter methylation was observed in 56/114 (49.1%) cases below 50 years while 59/114 (50.9%) cases are above 50 years. When we compare with the age of the patients below 50 years and above 50 years but no statistical difference was observed.
Figure 5.
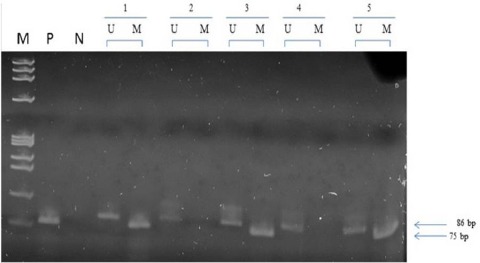
Representative Picture Showing the Promoter Methylation of BRCA1 Gene Tumor and Control Samples. M is the phi x 174 Hae III digested marker. P is SSs1 treated CpG methylated DNA used as positive control. N is negative control. U is unmethylated product (75bp). M is methylated product (86bp).
Menopausal status was studied in 114 cases, 48 (42.1%) pre-menopausal cases and 66 (59.2%) were postmenopausal cases. Correlation between menopausal status and BRCA1 promoter methylation it was not found to be statistical significant (p<0.84) (Table 2). Estrogen receptor was studied in 114 cases out of which 77(67.5%) were ER-positive and 37(32.5%) were ER-negative. No statistically significant difference was observed.
Table 2.
Comparison BRCA1 Promoter Methylation and Age, Menopausal Status, ER, PR and HER2 status (n=114)
| BRCA1 promoter methylation | p value | ||
|---|---|---|---|
| Methylation present | 68 (59.6%) | ||
| Methylation absent | 46(40.4%) | ||
| Age | BRCA1 promoter methylation | ||
| Below 50 years | 56 (49.1%) | ||
| Above 50 years | 59 (50.9%) | ||
| Menopausal status | Methylation Present | Methylation Absent | (p>0.8456) |
| Pre-menopausal 48 (42.1%) | 31 (64.6%) | 17 (35.4%) | |
| Post-menopausal 66 (57.9%) | 41 (62.1%) | 25 (37.9%) | |
| ER Expression | Methylation Present | Methylation Absent | (p>0.4068) |
| ER positive 77 (67.5%) | 47 (60.2%) | 30 (38.8%) | |
| ER negative 37 (32.5%) | 26(72.2%) | 11 (29.8%) | |
| PR Expression | Methylation Present | Methylation Absent | (p>0.5553) |
| PR positive 48 (42.1%) | 29 (60%) | 19(40%) | |
| PR negative 66 (57.9%) | 44(64.7%) | 22 (33.3%) | |
| HER 2 Expression | Methylation Present | Methylation Absent | (p>1.0000) |
| HER positive 43 (37.8%) | 27 (62.8%) | 16(37.2%) | |
| HER 2 negative 71 (62.2%) | 45 (63.3%) | 26 (36.7%) |
For PR 44/66 (64.7%) cases with PR negative status showed BRCA1 methylation while 22/66 (33.3%) were unmethylated. whereas 29/48 (60.4%) cases are PR positive were BRCA1 methylated and 19/48 (39.6%) are unmethylated but not statistically significant. Similarly, we check the difference between BRCA1 promoter methylation and other factors like histogical subtypes, tumor staging, in-situ component, lymph node, RT/CT status and HER2, no statistical association was observed.
Comparisons between BRCA1 protein expression and BRCA1 promoter methylation
BRCA1 protein expression was studied in 114 breast cancer cases, out of which 55/114 (48.2%) cases showed reduced or absent expression. Out of 55 cases, 34/55 (61.8%) cases showed promoter methylation. When we compared the status of 34 cases which showed BRCA1 promoter methylation, interestingly it was observed that 26 (76.5%) cases showed reduced or absent BRCA1 protein expression. However, the difference was statistical significant (p<0.001).
Discussion
Breast cancer is second most common cancer in the world and the leading cause of cancer-related death in women in both developed and developing countries (Jemal et al., 2011). Estrogen Receptor (ER), Progesterone Receptor (PR) and HER2 are used for prognostication, and to stratify patients for appropriately targeted therapies (Lidereau et al., 2000). Molecular profiling has provided evidence for this heterogeneity and thus there is a steady interest to identify new markers that will help in predicting prognosis and response to therapy.
Age is an important independent prognostic factor in breast cancer; younger age has been shown to be an adverse factor in general. Indian population is relatively young as compared to the aging of the western population and also considering the increased life expectancy of western population i.e. 75-80 years while in Indian population it is 65 years. Majority of cases (39.5%) were less than 50 years with a peak at 40-49 years and our observation also in agreement with other studies (Amirrad et al., 2005; Chopra et al., 2014)
In our study BRCA1 expression was reduced in 52.0% in premenopausal and 43.9% of postmenopausal cases, however, the difference was not statistically significant (p>0.52), the results were in agreement with the previous studies (Jasin, 1996). The most common histological subtype was invasive ductal Carcinoma (87.70%) and others cases are a lobular carcinoma, lobular, papillary, invasive medullary, metaplastic, Idc with lobular and malignant phyllodes. This is consistent with an Indian study which also showed that invasive ductal carcinoma was found to be the most common type (88%) (Leong et al., 2010).
The study showed 67.5% ER-positive and 42.1% PR-positive cases. 42.1% cases were double positive and 32.4% cases were a double negative for ER and PR expression. In the present study, 32.5% cases were ER-negative and 57.9% are PR-negative cases. The incidence of ER or PR negative tumors in western population is 17–37%, whereas in Indian studies the ER or PR negativity is somewhat higher around 55-80% (Quick et al., 2008). The reason for higher ER or PR negativity is suggested to be due to improper staining or suboptimal manual assays rather than the presence of genetic differences (Fanelli et al., 1996).
Navani and Bhaduri, (2005) in their study restained manually 37 ER-negative and PR-positive tumors using FDA approved automated technique and staining protocols similar to the manual assays found the majority (75.6%) of tumors were false negative. However in our study, not even a single case showing such discrepancy was found. Hereditary breast cancers are seen to have high cases of ER- negative cases and approximately 70% cases of sporadic breast cancers are ER-positive (Mukohara, 2011) and in the present study 67.50% cases were ER-positive consistent with previous studies. HER2 is over-expressed in 20–30% of breast cancer (Hu et al., 2006). In the present study 37.8% cases were seen to be HER2 positive. HER2 over-expression has been shown to be associated with partial resistance to endocrine treatment (Thakur et al., 1997). Trastuzumab (Herceptin) is a monoclonal antibody directed against HER2, thus useful in tumors showing HER2 over-expression (Thakur et al., 1997).
In our study, 51.37% staining remained to the nucleus, 2.7% localized to cytoplasm and 37.61% expressed BRCA1 in both nucleus and to the cytoplasm. Our observations were comparable with Lambie et al., (2003). In the present study BRCA1 expression was found to be reduced in 48.2% cases of 114 breast cancer cases and our results were in agreement with the other studies. Several studies observed that BRCA1 expression was reduced in 30 – 83% (Dinesh et al., 2006; Hedau et al., 2011). This reduced expression in breast cancer cases suggests that BRCA1 may have an important role in sporadic breast cancer.
Various studies have shown conflicting results. A study by Hedau et al found reduced BRCA1 expression in post-menopausal women (57.5%) (Hedau et al., 2015). However in our study 43.9% cases of postmenopausal females had reduced BRCA1 expression which was not found to be statistically significant. The basic mechanisms are responsible for the conflicts in results may be because of estrogen stimulated increase in BRCA1 expression in older females which in turn may be due to presence of transformed cells with impaired function which are sensitive to proliferative effects of estrogen. However, the function of BRCA1 may be impaired in them due to increased methylation which is related to aging (Shastry and Yardley, 2013). The fact that BRCA1 inhibits a cellular response to estrogens suggests more complex interactions (Mukohara, 2011).
BRCA1 Promoter methylation
Loss of function of BRCA1 cannot be explained on the basis of BRCA1 mutation, since mutations of BRCA1 are rare in sporadic breast cancer, thus suggesting some epigenetic mechanisms (Hsu et al., 2013). The decrease in expression of the BRCA1 protein in our study could be due to methylation of the BRCA1 promoter region. The present study shows that BRCA1 promoter region is methylated in 59.6% cases which is higher than previously reported frequencies (Teschendorff et al., 2010; Bosviel et al., 2012). BRCA1 expression was seen to be absent or markedly decreased in 50% cases of all promoter methylated cases suggesting epigenetic silencing in these.
BRCA1 was methylated in 60.2% cases of ER-positive tumors and 72.2% cases of ER-negative tumors. On the other hand our study showed that BRCA1 was methylated in 63.0% cases of PR positive tumors and was methylated in 64.7% cases of PR negative tumors. However, the present study did not find the differences to have any statistical significance which is consistent with findings by others35-36. The study shows that high- grade ER-positive subset is frequently BRCA1 methylated in agreement with the findings by Matros et al in a hierarchical gene expression clustering analysis to find a role of methylation in inactivation in sporadic cases (Arpino et al., 2004).
There was a subset of high-grade methylated tumors (four cases) showing age less than 50 years in three out of four cases, tumor size less than 5 cm in three out of four cases, showing lymph node involvement in three out of four cases. Histologically one case was of medullary and one of metaplastic cancer and rest two Idc. All had the presence of in situ component, two cases showing ER expression and all four negative for PR expression. In two cases HER2 was absent, in one equivocal and one positive. Three cases showed moderate nuclear BRCA1 expression and one strong nuclear expression. The finding that in younger patients with BRCA1 methylated high grade smaller size tumors having in situ component at diagnosis with frequent hormonal receptors negative tumors there was no reduction of expression of BRCA1, suggests that BRCA1 may be responsible for breast cancer in these cases through the presence of non-functional truncated BRCA1 protein. BRCA1 methylation was seen in 63.3% of HER2-negative cases and 62.8% cases of HER2 positive cases. The results were not statistically significant compared to those in unmethylated cases as shown by others (Arpino et al., 2004; Szyf, 2012).
However in our study there was a subset (ER, PR and HER2) which showed presence BRCA1 methylation along with BRCA1 expression positive cases. They constituted 36/114 (31.57%) of cases studied for methylation and BRCA1 expression. In this group, mechanisms other than BRCA1 carcinogenesis may be responsible for normal BRCA1 expression or there may be the presence of abnormal or truncated protein of BRCA1 which is non-functional. Aberrant methylation is a common finding in cancer cells (Dakubo et al., 2007) and it is shown to cause down-regulation of many tumor suppressor genes in human cancers. A study by Magdinier et al have shown that DNA methylation in breast cancer may not be associated with down-regulation of BRCA1, alternatively, methylation may regulate pathways which are different from BRCA1 and are yet to be discovered (Sharma et al., 2010).
In conclusion, reduced BRCA1 expression was found in 48.2% of breast cancer and showed significant association with ER-negative status and PR-negative status. The BRCA1 expression is not associated with age, menopausal status, side, in-situ component, pathological tumor stage (T), lymph node status, neo-adjuvant treatment, HER2/neu expression. BRCA1 promoter methylation was found in 59.6% of breast cancer in our study and these associations with postmenopausal status with more percentage of cases showing methylation in postmenopausal females.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank Science and Engineering Research Board (SERB), UCMS and ICMR for their support.
References
- Amirrad M, AI-Mulla F, Varadharaj G, et al. BRCA1 gene expression in breast cancer in Kuwait:Correlation with prognostic parameters. Med Princ Pract Int Kuwait Univ Health Sci Cent. 2005;14:67–72. doi: 10.1159/000083913. [DOI] [PubMed] [Google Scholar]
- Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast:tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:149–56. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosviel R, Garcia S, Lavediaux G, et al. BRCA1 promoter methylation in peripheral blood DNA was identified in sporadic breast cancer and controls. Cancer Epidemiol. 2012;36:177–82. doi: 10.1016/j.canep.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Chopra B, Kaur V, Singh K, et al. Age shift:Breast cancer is occurring in younger age groups –Is it true? Clin Cancer Investig J. 2014;3:526. [Google Scholar]
- Dakubo GD, Jakupciak JP, Birch-Machin MA, Parr RL. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;7:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh KP, Devaraj H, Murugan V, et al. Analysis of loss of heterozygosity and immunohistochemistry in BRCA1 gene in sporadic breast cancer. Mol Cell Biochem. 2006;287:177–83. doi: 10.1007/s11010-005-9097-z. [DOI] [PubMed] [Google Scholar]
- Elledge RM, Green S, Pugh R, et al. Estrogen receptor (ER) and progesterone receptor (PR), by ligand-binding assay compared with ER, PGR and PS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer:a southwest oncology group study. Int J Cancer. 2000;89:111–7. [PubMed] [Google Scholar]
- Fanelli MA, Vargas-Roig LM, Gago FE, et al. Estrogen receptors, progesterone receptors, and cell proliferation in human breast cancer. Breast Cancer Res Treat. 1996;37:217–28. doi: 10.1007/BF01806503. [DOI] [PubMed] [Google Scholar]
- Hedau S, Kumar U, Hussain S, et al. Breast cancer and human papillomavirus infection:no evidence of HPV etiology of breast cancer in Indian women. BMC Cancer. 2011;1:11–27. doi: 10.1186/1471-2407-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedau S, Batra M, Singh UR, et al. Expression of BRCA1 and BRCA2 proteins and their correlation with clinical staging in breast cancer. J Cancer Res Ther. 2015;11:158–63. doi: 10.4103/0973-1482.140985. [DOI] [PubMed] [Google Scholar]
- Hsu NC, Huang Y-F, Yokoyama KK, et al. Methylation of BRCA1 promoter region is associated with unfavorable prognosis in women with early-stage breast cancer. PLoS One. 2013;8:e56256. doi: 10.1371/journal.pone.0056256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M. Genetic manipulation of genomes with rarecutting endonucleases. Trends Genet. 1996;12:224–8. doi: 10.1016/0168-9525(96)10019-6. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. Ca Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Lambie H, Miremadi A, Pinder SE, et al. Prognostic significance of BRCA1 expression in sporadic breast carcinomas. J Pathol. 2003;200:207–13. doi: 10.1002/path.1348. [DOI] [PubMed] [Google Scholar]
- Leong SPL, Shen Z-Z, Liu T-J, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34:2308–24. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidereau R, Eisinger F, Champème MH, et al. Major improvement in the efficacy of BRCA1 mutation screening using morphoclinical features of breast cancer. Cancer Res. 2000;60:1206–10. [PubMed] [Google Scholar]
- Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Fukutomi T, Asada K, et al. Promoter hypermethylation and post-transcriptional mechanisms for reduced BRCA1 immunoreactivity in sporadic human breast cancers. Jpn J Clin Oncol. 2002;32:79–84. doi: 10.1093/jjco/hyf020. [DOI] [PubMed] [Google Scholar]
- Mukohara T. Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci. 2011;102:1–8. doi: 10.1111/j.1349-7006.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- Navani S, Bhaduri AS. High incidence of oestrogen receptor negative progesterone receptor positive phenotype in Indian breast cancer:fact or fiction? Indian J Pathol Microbiol. 2005;48:199–201. [PubMed] [Google Scholar]
- Quick EL, Parry EM, Parry JM. Do oestrogens induce chromosome specific aneuploidy in vitro, similar to the pattern of aneuploidy seen in breast cancer. Mutat Res. 2008;12:46–55. doi: 10.1016/j.mrgentox.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Rakha EA, El-Sheikh SE, Kandil MA, et al. Expression of BRCA1 protein in breast cancer and its prognostic significance. Hum Pathol. 2008;39:857–65. doi: 10.1016/j.humpath.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Sakakibara S, Imai T, Hamaguchi K, et al. Mouse-Musashi-1 , a neural RNA-binding protein Highly enriched in the mammalian CNS. Stem Cell. 1996;242:230–42. doi: 10.1006/dbio.1996.0130. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastry M, Yardley DA. Updates in the treatment of basal/triple-negative breast cancer. Curr Opin Obstet Gynecol. 2013;25:40–8. doi: 10.1097/GCO.0b013e32835c1633. [DOI] [PubMed] [Google Scholar]
- Szyf M. DNA methylation signatures for breast cancer classification and prognosis. Genome Med. 2012;4:26. doi: 10.1186/gm325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff AE, Menon U, Gentry-Maharaj A, et al. Age-dependent DNA methylation of genes that are suppressed in stem cell is a hallmark of cancer. Genome Res. 2010;20:440–6. doi: 10.1101/gr.103606.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S, Zhang HB, Peng Y, et al. Localization of BRCA1 and a splice variant identifies the nuclear localization signal. Mol Cell Biol. 1997;17:444–52. doi: 10.1128/mcb.17.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulchin N, Ornstein L, Dikman S, et al. Localization of BRCA1 protein in breast cancer tissue and cell lines with mutations. Cancer Cell Int. 2013;13:70. doi: 10.1186/1475-2867-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer:prognosis and prediction. Endocr Relat Cancer. 2010;17:245–62. doi: 10.1677/ERC-10-0136. [DOI] [PubMed] [Google Scholar]
- WHO. International Agency for Research on Cancer. 2012 [Google Scholar]
- Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–92. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, et al. Identifying tumor suppressors in genetic mosaics :the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–63. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]


