Abstract
Background:
The effect of body mass index (BMI) on postoperative survival in non-small cell lung cancer (NSCLC) has been controversial. We retrospectively analysed the effect of preoperative BMI on postoperative outcomes of NSCLC surgery.
Methods:
Consecutive 384 NSCLC patients were enrolled. Patients were subdivided into 3 groups: low BMI group (BMI<18.5 kg/m2), normal BMI group (BMI=18.5-24.0 kg/m2) and high BMI group (BMI>24.0 kg/m2). The prognostic significance of BMI was examined retrospectively.
Results:
The 5-year survival of patients with low, normal and high BMI groups were 46.3%, 74.3% and 84.3%, respectively. The low BMI group had a poorer prognosis than the other groups (p<0.001). The survival of high BMI group had a more favorable trend than that of normal BMI group, but this did not reach statistical significance (p=0.057). On multivariate analysis, significant risk factors for cancer-specific survival were male gender (p=0.0061), non-adenocarcinoma histology (p=0.0003), pN1-2 status (p=0.0007), high serum CEA level (p<0.0001) and low BMI (low vs. others: p <0.0001).
Conclusions:
Preoperative BMI is an independent prognostic factor for NSCLC patients after surgical resection, with low BMI patients having an unfavorable prognosis.
Keywords: Body mass index, non-small cell lung cancer, prognosis
Introduction
Nutritional status, generally evaluated using body mass index (BMI), is known to be related to several diseases. It was commonly believed that obesity (higher BMI) could increase the morbidity and mortality of surgical patients. For patients with non-small cell lung cancer (NSCLC), some previous investigations showed that high BMI is a risk factor for cardiopulmonary complications after surgery (Petrella et al., 2011, Launer et al., 2013, Thomas et al., 2014). On the other hand, others have denied this result (Dhakal et al., 2013, Smith et al., 2007, Attaran et al., 2012).
With regard to postsurgical survival, St Julien et al. showed worse survival in patients with higher BMI (St Julien et al., 2012), while Win et al. found that high BMI had no impact on NSCLC prognosis (Win et al., 2008). To our surprise, a new discovery termed the ‘obesity paradox’, which refers to a better prognosis in obese patients compared to normal/underweight patients, has been recently identified in surgical populations (Valentijn et al., 2013). Recent investigations (St Julien et al., 2012, Matsunaga et al., 2015, Yang et al., 2011) found the prognostic roles of high BMI and suggested that the obesity paradox may really exist in patients having NSCLC surgery. However, the mechanism by which being high BMI might prolong patient survival is still not well understood. In general, it has been believed that malnutrition is associated with an increased risk of poor tissue healing, impaired immune function, and respiratory muscle dysfunction (Bashir et al., 1990, Win et al., 2008). Regarding NSCLC patients with lower BMI, previous investigations showed that low BMI is a risk factor for surgical outcome (Tewari et al., 2007, Nakagawa et al., 2016, Suzuki et al., 2016), while another have denied this result (Win et al., 2007). No consensus has been reached on the prognostic value of BMI in NSCLC surgery. Therefore, the nutrition assessment is generally not a routine part of the preoperative assessment.
In the present study, we reviewed our patients with NSCLC to identify the prognostic significance of BMI in NSCLC surgery patients.
Materials and Methods
Patients and Methods
This retrospective study had institutional review board approval, and the need to obtain patient consent was waived. Consecutive NSCLC patients who underwent curative surgery from 2008 to 2013 in our hospital were enrolled into the present retrospective study. All procedures were attended using general anesthesia with selective intubation and via thoracotomy. Lobectomy was considered and performed in any case that could be completely resected based on respiratory function, performance status, and comorbidities in every single patient. Radical lymph node resection was always performed. At the end of the procedure, one intercostal drainage tube was generally placed in the pleural cavity. The drainages were removed in the absence of air-leaks and/or pneumothorax on chest X-ray. In the present study, patients who died of other diseases or were lost to follow-up were excluded. The collected records of 384 consecutive NSCLC patients were reviewed retrospectively.
The preoperative BMI was calculated as weight in kilograms divided by height in meters squared. The following category was used: low BMI group (BMI<18.5 kg/m2), normal BMI group (BMI=18.5-24.0 kg/m2) and high BMI group (BMI>24.0 kg/m2). The clinicopathologcal factors of patients were shown in Table 1.
Table 1.
Clinical Characteristics of Patents
| Low BMI |
Normal BMI |
High BMI |
Total | p Value |
|
|---|---|---|---|---|---|
| Age | |||||
| ≤65 | 19 | 68 | 38 | 125 | 0.689 |
| >65 | 40 | 151 | 68 | 259 | |
| Gender | |||||
| Male | 32 | 122 | 44 | 198 | 0.051 |
| Female | 27 | 97 | 62 | 186 | |
| Smoking status | |||||
| Never | 26 | 94 | 52 | 172 | 0.577 |
| Current/former | 33 | 125 | 54 | 212 | |
| Histology | |||||
| Adenocarcinoma | 42 | 162 | 92 | 296 | 0.013 |
| Others | 17 | 57 | 14 | 88 | |
| pStage | |||||
| I | 42 | 169 | 85 | 296 | 0.428 |
| II-III | 17 | 50 | 21 | 88 | |
| pT status | |||||
| pT1 | 33 | 154 | 72 | 259 | 0.12 |
| pT2-3 | 26 | 65 | 34 | 125 | |
| pN status | |||||
| pN0 | 51 | 182 | 93 | 326 | 0.509 |
| pN1-2 | 8 | 37 | 13 | 58 | |
| CEA | |||||
| Normal | 39 | 162 | 81 | 282 | 0.355 |
| High | 20 | 57 | 25 | 102 |
BMI, body mass index; CEA, carcinoembryonic antigen
Chi-square test was used to determine the association among BMI groups. Cumulative survival curves after surgery were calculated using the Kaplan–Meier method and differences were evaluated using the log-rank test. Univariate analysis and multivariate analysis using Cox’s proportional hazards regression model were used to identify the prognostic factors. Differences were considered significant when the p value was <0.05. All statistical analyses were performed using JMP (SAS Institute Inc., Cary, NC, USA).
Results
Table 1 shows baseline characteristics of the study patients. Among these patients, 15.4 % were categorized as low BMI, 57% as normal BMI, and 27.6% as high BMI. Age, gender, smoking status, pStage, pT status, pN status, serum CEA level were similar between BMI groups. However, high BMI group had a statitically significant association with increasing adenocarcinoma histology (p=0.013), but this difference was not found in low BMI group.
The range of follow-up duration was 39-109 months. As shown in Figure 1, comparison of postoperative survival curves according to BMI group showed that there was a significant difference for patients’ survival (p<0.001). The 5-year survival of patients with low, normal and high BMI groups were 46.3%, 74.3% and 84.3%, respectively. The low BMI group had a significantly worse survival than the normal BMI and high BMI groups (p<0.001). Furthermore, there was a trend that the survival of high BMI group is more favorable than that of normal BMI group, but this did not reach statistical significance (p=0.057).
Figure 1.
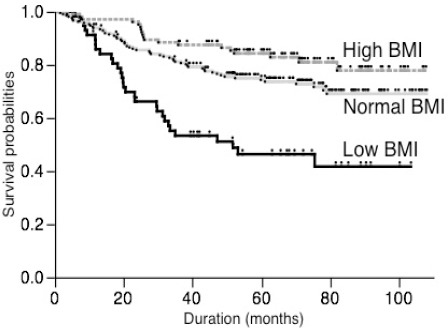
Comparison of Survival Curves According to the BMI Groups
Univariate analysis identified that male gender (p<0.0001), current/former smoking status (p<0.0001), non-adenocarcinoma histology (p<0.0001), pT2-3 status (p<0.0001), pN1-2 status (p<0.0001), high serum CEA level (p<0.0001), low BMI (low vs. others: p <0.0001) and high BMI (high vs. others: p =0.002) as significant factors for poor survival (Table 2).
Table 2.
Univariate analysis
| Favorable | Unfavorable | Hazard ratio | p Value | 95.0% confidence interval | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | ≤65 | >65 | 0.771 | 0.225 | 0.495 | 1.169 |
| Gender | Fenale | Male | 0.34 | <0.001 | 0.219 | 0.513 |
| Smoking | Never | Current/Former | 0.348 | <0.001 | 0.222 | 0.529 |
| Histology | Adeno | Others | 0.284 | <0.001 | 0.193 | 0.42 |
| pT status | pT1 | pT2-3 | 0.399 | <0.001 | 0.272 | 0.585 |
| pN status | pN0 | pN1-2 | 0.361 | <0.001 | 0.238 | 0.563 |
| CEA | Normal | High | 0.378 | <0.001 | 0.254 | 0.566 |
| BMI | Others | Low | 0.347 | <0.001 | 0.23 | 0.539 |
| High | Others | 0.489 | 0.002 | 0.289 | 0.785 | |
CEA, carcinoembryonic antigen; BMI, body mass index
Multivariate analysis using a Cox proportional hazards model showed that male gender (p=0.0061), non-adenocarcinoma histology (p=0.0003), pN1-2 status (p=0.0007), high serum CEA level (p<0.0001) and low BMI (low vs. others: p <0.0001) was an independent predictor of poor prognosis (Table 3). Although the difference of patients’ survival between high BMI and others did not reach statistical significance, low BMI was a statistically significant independent unfavorable factor for NSCLC patients’ prognosis after surgery.
Table 3.
Multivariate Analysis
| Favorable | Unfavorable | Hazard ratio | p Value | 95.0% confidence interval | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Gender | Female | Male | 0.44 | 0.007 | 0.233 | 0.806 |
| Smoking | Never | Current/Former | 0.942 | 0.856 | 0.488 | 1.781 |
| HIstology | Adeno | Others | 0.449 | <0.001 | 0.291 | 0.693 |
| pT1 status | pT1 | pT2-3 | 0.786 | 0.27 | 0.514 | 1.207 |
| pN status | pN0 | pN1-2 | 0.436 | 0.001 | 0.272 | 0.711 |
| CEA | Normal | High | 0.384 | <0.001 | 0.258 | 0.573 |
| BMI | Others | Low | 0.391 | <0.001 | 0.251 | 0.622 |
| High | Others | 0.716 | 0.2 | 0.413 | 1.187 | |
CEA, carcinoembryonic antigen; BMI, body mass index
Discussion
In addition to cancer stage and histological type, we observed that low BMI was crudely associated with poorer prognosis after surgery for patients with NSCLC. This result was consistent with previous investigations (Tewari et al., 2007, Nakagawa et al., 2016, Suzuki et al., 2016). Tewari et al. investigated 642 NSCLC patients undergoing lobectomy and reported that nutritional status does not appear to significantly influence immediate outcomes following lobectomy for NSCLC, but a low BMI was a negative predictor of long-term survival independent of tumor extension and stage (Tewari et al., 2007). Nakagawa et al., (2016) examined 1311 NSCLC patients and showed that low BMI had a negative effect on surgical outcomes. Suzuki et al., (2016) investigated 137 NSCLC patients who underwent lung resection with curative intent and found that patients with sarcopenia, who were more likely to have a low BMI, had a poor outcome with completely resected early stage NSCLC (Suzuki et al., 2016). Therefore, nutrition assessment should be considered as a routine part of the preoperative assessment. However, the underlying mechanism of this effect is unknown in detail. There are no substantial reports on the effects of low BMI specific to NSCLC surgery. The nutritional depletion might be associated with an increased risk of respiratory muscle weakness, poor tissue healing and altered metabolism (Petrella et al., 2011, Launer et al., 2013, Thomas et al., 2014, Tewari et al., 2007, Nakagawa et al., 2016, Suzuki et al., 2016). It is easy to understand that the respiratory muscle weakness, poor tissue healing and altered metabolism due to the nutritional depletion might increase the early postoperative complications. However, we believe that these mechanisms can not fully explain the reason for the relationship between low BMI and long term survival after NSCLC surgery. One of possible reasons is that the impaired immune function due to nutritional depletion might possibly fail the boosting of the ability of the body’s immune system in cancer removal. In contrast to these investigations, Win et al., (2007) found that there was no association between low BMI and postoperative death or poor surgical outcome in patients with operable NSCLC. However, in their study, there are only 7 (4.8%) patients had low BMI (Win et al., 2007).
There is a growing interest in the relationship between high BMI and favorable surgical survival after NSCLC surgery (Attaran et al., 2012, St Julien et al., 2012, Matsunaga et al., 2015, Yang et al., 2011). The underlying mechanism that may mediate this beneficial high BMI effect is unknown. One common hypothesis is that high BMI patients had a better nutritional status. High BMI patients can better store nutrients to resist surgical interventions compared to normal/low BMI patients (Bundhun et al., 2015). Furthermore, high BMI patients are generally treated at an early age with medicines to control blood pressure and prevent hyperglycemia. This situation may be an another reason for the obesity paradox. The present results showed that high BMI group had a trend of favorable prognosis than normal BMI, but failed to find a statistical difference. One of possible reasons for this discrepancy is that our study population is Japanese patients. Yoshiike et al., (1998) reported that the standardized prevalence of obesity (BMI > or = 30.0) in Japanese adults was quite low compared with the data in western populations. In the present series, there are only 5/384 (1.3%) patients with BMI > or = 30.0. Previous studied classified their patients into four groups as follows: underweight, normal weight, overweight and obese (Nakagawa et al., 2016, Thomas et al., 2014). However, we subdivided our patients as low, normal and high BMI groups because of very small number of patients with obese. Therefore, there is a possibility that we might fail to find the obesity paradox because of small number of obese patients.
Several limitations of the analysis should be considered. One limitation is that this was a retrospective and single institute study. Furthermore, as commented above, there are small number of patients with obese. In addition, the number of study patients was rather small. Therefore, further studies using large population in this area are warranted.
In conclusions, low BMI is an independent risk factors for poor surgical outcomes in NSCLC patients. Although we failed to find the obesity paradox, nutritional status was an important factor in determining postoperative survival after NSCLC surgery.
References
- Attaran S, McShane J, Whittle I, et al. A propensity-matched comparison of survival after lung resection in patients with a high versus low body mass index. Eur J Cardiothorac Surg. 2012;42:653–8. doi: 10.1093/ejcts/ezs135. [DOI] [PubMed] [Google Scholar]
- Bashir Y, Graham TR, Torrance A, et al. Nutritional state of patients with lung cancer undergoing thoracotomy. Thorax. 1990;45:183–6. doi: 10.1136/thx.45.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundhun PK, Li N, Chen MH. Does an obesity paradox really exist after cardiovascular intervention?:A systematic review and meta-analysis of randomized controlled trials and observational studies. Medicine (Baltimore) 2015;94:e1910. doi: 10.1097/MD.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal B, Eastwood D, Sukumaran S, et al. Morbidities of lung cancer surgery in obese patients. J Thorac Cardiovasc Surg. 2013;146:379–84. doi: 10.1016/j.jtcvs.2013.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer H, Nguyen DV, Cooke DT. National perioperative outcomes of pulmonary lobectomy for cancer in the obese patient:a propensity score matched analysis. J Thorac Cardiovasc Surg. 2013;145:1312–8. doi: 10.1016/j.jtcvs.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Suzuki K, Imashimizu K, et al. Body mass index as a prognostic factor in resected lung cancer:obesity or underweight, which is the risk factor? Thorac Cardiovasc Surg. 2015;63:551–7. doi: 10.1055/s-0035-1554964. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Toyazaki T, Chiba N, et al. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact Cardiovasc Thorac Surg. 2016;23:560–6. doi: 10.1093/icvts/ivw175. [DOI] [PubMed] [Google Scholar]
- Petrella F, Radice D, Borri A, et al. The impact of preoperative body mass index on respiratory complications after pneumonectomy for non-small-cell lung cancer. Results from a series of 154 consecutive standard pneumonectomies. Eur J Cardiothorac Surg. 2011;39:738–44. doi: 10.1016/j.ejcts.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Smith PW, Wang H, Gazoni LM, et al. Obesity does not increase complications after anatomic resection for non-small cell lung cancer. Ann Thorac Surg. 2007;84:1098–105. doi: 10.1016/j.athoracsur.2007.04.033. [DOI] [PubMed] [Google Scholar]
- St Julien JB, Aldrich MC, Sheng S, et al. Obesity increases operating room time for lobectomy in the society of thoracic surgeons database. Ann Thorac Surg. 2012;94:1841–7. doi: 10.1016/j.athoracsur.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Okamoto T, Fujishita T, et al. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer. 2016;101:92–7. doi: 10.1016/j.lungcan.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Tewari N, Martin-Ucar AE, Black E, et al. Nutritional status affects long term survival after lobectomy for lung cancer. Lung Cancer. 2007;57:389–94. doi: 10.1016/j.lungcan.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Thomas PA, Berbis J, Falcoz PE, et al. National perioperative outcomes of pulmonary lobectomy for cancer:the influence of nutritional status. Eur J Cardiothorac Surg. 2014;45:652–9. doi: 10.1093/ejcts/ezt452. [DOI] [PubMed] [Google Scholar]
- Valentijn TM, Galal W, Tjeertes EK, et al. The obesity paradox in the surgical population. Surgeon. 2013;11:169–76. doi: 10.1016/j.surge.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Win T, Ritchie AJ, Wells FC, et al. The incidence and impact of low body mass index on patients with operable lung cancer. Clin Nutr. 2007;26:440–3. doi: 10.1016/j.clnu.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Win T, Sharples L, Groves AM, et al. Predicting survival in potentially curable lung cancer patients. Lung. 2008;186:97–102. doi: 10.1007/s00408-007-9067-1. [DOI] [PubMed] [Google Scholar]
- Yang R, Cheung MC, Pedroso FE, et al. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res. 2011;170:e75–83. doi: 10.1016/j.jss.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiike N, Matsumura Y, Zaman MM, et al. Descriptive epidemiology of body mass index in Japanese adults in a representative sample from the national nutrition survey 1990-1994. Int J Obes Relat Metab Disord. 1998;22:684–7. doi: 10.1038/sj.ijo.0800651. [DOI] [PubMed] [Google Scholar]


