Abstract
Background:
Despite advances in cancer disease prevention, diagnosis, and treatment patients with cancer suffer from a variety of sometimes severe physical and psychological symptoms regardless of the stage of the disease. The aim is to determine the relationship of antecedent factors and mediating variables to the quality of life (QOL) of patients with cancer.
Materials and Methods:
The study included 341 patients with cancer and symptoms. Data collection used the Cancer Symptom Scale, State-Trait Anxiety Inventory, Centers for Epidemiologic Study-Depression, Cancer Behavior Inventory, Multidimensional Quality of Life Index, and a Demographic Form.
Results:
A multiple regression equation containing all the variables explained 68% of the variance in QOL. Overall four variables accounted for the majority of the total variance: anxiety, depression, self-efficacy, and symptom severity. Three of these variables were mediating variables. Of the antecedent factors symptom severity had a significant indirect effect on QOL through the mediating variables. Symptom severity also had direct effect on QOL.
Conclusion:
Data indicate that anxiety, depression, and self-efficacy play major roles in determining the perception of QOL of cancer patients. These mediating variables either buffered or enhanced the impact of the antecedent factors of symptom severity on QOL. Nursing interventions should focus on enhancing self-efficacy. Nurses can use health promoting programs to assist patients who are physically impaired. Further research should be aimed at identifying other influential variables, with the ultimate goal of developing interventions to aid patients in their efforts to maintain their QOL while living with cancer.
Keywords: Quality of life, self-efficacy, path analysis, cancer
Introduction
Despite advances in cancer disease prevention, diagnosis, and treatment, patients with cancer suffer from a variety of sometimes severe physical and psychological symptoms regardless of the stage of the disease (Naughton and Hansi, 2007; Portenoy et al., 1994). Some symptoms may be directly associated with progression of the disease, whereas others are associated with the treatment (Chang, Hwang et al., 2000; Donnelly et al., 1995). Such symptoms can negatively influence quality of life (QOL) and greatly increase patients’ distress (Omran et al., 2012; Landers et al., 2011; Jim et al., 2008). Effectiveness of treatment strategies should be judged not only in terms of increasing chances of cure but also in terms of diminishing the impact of treatment on QOL (de Graff et al., 2000). Assessing QOL can be seen as a step toward a more comprehensive evaluation of patients with cancer. In fact, QOL is the center core in providing palliative care (Finley and Dunlop, 1994).
Quality of life is a complex, multidimensional construct (Bindewald et al., 2007) that includes the satisfaction of physical, social, and emotional needs (Murphy et al., 2007; Peplau, 1994). It relates to psychosocial as well as physical well-being. QOL is a crucial factor in health care policy and decision making (De Aguiar et al., 2014; Li et al., 2012). This is true in patients with cancer, as in any other chronic disease, because the goals of health care providers are focused on returning the patient to a life style that is not diminished by the illness or its treatment (Williams et al., 2001; Hopwood and Stephens, 2000). Researchers have indicated that QOL is primarily a perception, an idea that individuals form after sensing, observing, or recognizing intuitively the meaning of something that has been experienced. It is not the experience per se, but rather an opinion or judgment that sums the essence of a situation or experience, a series of events, or the current view about one’s life in part or as whole in a given period of time (Mount, 1993; Mystakidou et al., 2008).
The experience of living with cancer from the time of diagnosis, through treatment, and survival is troubled with psychological distress (Gill et al., 2012; Mount, 1993; Chang et al, 1998; Finley and Dunlop, 1994). Kurtz and colleagues (2001) indicated that psychological distress is particularly associated with longer hospital stays, poor treatment adherence, reduced self-care abilities, and shorter survival. Although it may not be always possible to change the course of cancer and its treatment outcomes, changing the patient’s perception of control (his/her ability to cope with illness or other stressful events) has been associated with positive psychosocial adjustments including less anxiety and depression (Monroe and Oliviere, 2006) and improved adjustments to the situation (Thompson et al., 1993).
Studies on self-efficacy or ‘a belief in one’s capability to organize and execute the courses of action required to manage prospective situations” (p.2) (Bandura, 1997) is related to psychological well-being in patients with cancer (Cunningham et al., 1991; Bandura, 1997). A study of a heterogeneous group of patients with cancer, revealed that maintaining a sense of self efficacy, enhances an individual’s perception of a situation by decreasing depression and anxiety (Maciejewski et al., 2007). In fact, patients with higher coping self-efficacy are more likely to participate in effective strategies in attaining desired psychological (improved adjustments and QOL) and medical outcomes (fewer intense symptoms and side effects) compared with those with lower self-efficacy (Bandura, 1997). Self-efficacy for managing symptoms and function may be crucial to a patient’s ability to manage physical and psychological encounters of cancer.
Because cancer and treatment related symptoms can significantly increase psychological distress and alter patient’s QOL (Portnoy et al., 1994), assessment and management at all stages of illness is critical (Trammer et al., 2003). Further, whether and how self-efficacy influences QOL of patients with cancer needs to be explored. We anticipate that a greater perception of control over one’s life would result in enhanced QOL. Therefore, the purpose of this study was to determine the relationship of antecedent factors (symptoms severity) and mediating variables (anxiety, depression, and self-efficacy) to the QOL of patients with cancer. This study will contribute to the understanding of the influence of symptoms severity, anxiety, depression, and self-efficacy on QOL.
The study addressed the following research hypotheses
Hypothesis 1: Antecedent factors (symptoms severity) have an indirect influence (via mediating variables) on QOL of patients with cancer.
Hypothesis 2: the mediating variables (i.e., anxiety, depression, and self-efficacy) have a direct influence on QOL of patients with cancer.
Sub hypotheses
H.2-1- Anxiety is a significant predictor of QOL
H.2-2- Depression is a significant predictor of QOL
H.2-3- Self- efficacy is a significant predictor of QOL
Assumptions: (1) QOL is a multidimensional construct, (2) QOL is continuous and ongoing response to events that affect the individual, (3) the participants answered truthfully and to the best of their ability, and (4) QOL, anxiety, depression, and self-efficacy can be measured.
Theoretical Framework
The evidence of individual differences in adjustment to a life event, such as the experience of living with cancer, are consistent with the cognitive model of stress, coping, and adaptation developed by Lazarus and Folkman (1984). This model proposes that it is the interaction between an event and a susceptible person, not the event itself that determines whether the situation produces stress or not. The person-environment transaction is mediated by the individual’s cognitive appraisal of the situation and by the availability of coping resources. Positive beliefs about self and a sense of personal control are resources used by patients for effective coping. The outcome of this coping includes adaptation in social functioning, life satisfaction, and somatic health. Lazarus and Folkman recognized that QOL is connected to the ways that people cope with stress and that a basic outcome of the coping process is being able to function at work and in social situations and having good life satisfaction and somatic health. Life satisfaction is often used synonymously with QOL.
The variable of symptoms severity were chosen to represent the antecedent factors (events/stressors) in this study. Anxiety, depression, and self-efficacy were identified as coping resources (mediating variables) used by patients with cancer. Adaptation was represented by the outcome variable, QOL.
Lazarus and Folkman framework (1984), and their description of the antecedent factors, mediating variables, and adaptive outcomes as well as the research on QOL guided variable selection for this study and placement of the variables into a causal model. Expected relationships between the antecedent factors and the mediating variables are shown in Figure 1. The direct relationships within the model are represented with unidirectional arrows and plus and minus signs indicating the nature of the hypothesized relationships. The model proposed that anxiety, depression, and self-efficacy directly affect QOL of patients with cancer, as well as mediating the effects of antecedent factors (the disease variable) on this outcome.
Figure 1.
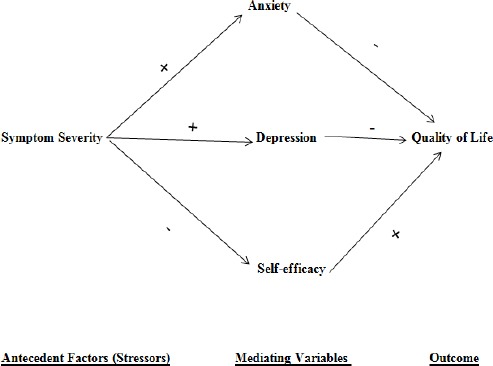
Hypothesized Causal Model of Quality of Life in Patients with Cancer
Path analysis was used to determine and test the significance of the path coefficients. Causal modeling is a technique used to evaluate the congruency of literature of the empirical data to the model. For a path analysis to be effective, the investigator must make the theoretical formulation very clear.
Materials and Methods
Sample
The data for this study was collected from the outpatient clinics at an NCI-designated comprehensive cancer center in Florida. A sample of 341 patients was accrued over 24 months. Inclusion criteria: Based on previous studies, patients with any type of cancer who have scores at baseline on intensity, distress and/or interference >4 on at least two symptoms were eligible for the study. Research has shown that patients with moderate to high symptom intensity, distress or interference are more likely to benefit from psychoeducational interventions (Given et al., 2004). Patients were adults, with cancer, able to read and understand English, able to pass mental status screening, and have functional status at the level of ECOG 3 or lower and mental status scores of >8. Exclusion criteria: Patients who had had surgery within the past six weeks were excluded, as were patients who were in hospice care or were confused or likely to die within 3 months.
Instruments
The study instruments included the Cancer Symptom Scale, State-Trait Anxiety Inventory, the Centers for Epidemiologic Study-Depression, the Cancer Behavior Inventory, the Multidimensional Quality of Life Index, and a Demographic Data Form.
Cancer Symptom Scale (CSS)
Symptoms are conceptualized to be patient stressors. Symptom occurrence, intensity, frequency and interference were assessed using the Cancer Symptom Scale (CSS). Miaskowski and colleagues (Miaskowski et al., 2007) called for studies of cancer patient symptoms that include measures of interference of symptoms with daily life, mood and quality of life. Although symptom intensity has been the focus of many investigators, symptom distress is gaining increasing attention in the literature. While correlated with intensity, symptom distress is not the same construct and deserves separate assessment (Cleeland et al., 2000; Linden and Girgid, 2012; McMillan and Small, 2002). Symptom interference with activities of daily living has been evaluated in recent studies (Tofthagen and McMillan, 2010; Given et al., 2008; Soltow, Given and Given, 2010) and may have a significant impact on quality of life. Beck and colleagues (Beck et al, 2005) made the case that frequency of symptoms is important. Thus, all of these dimensions were assessed as patient stressors using the CSS with a list of 35 symptoms. Like similar scales in the literature (Cleeland et al., 2000; Tofthagen and McMillan, 2010; Bruera et al., 1994; Portenoy et al., 1994), this scale allows the patient to identify whether a symptom has occurred in the past week (yes/no), and only if the response is “yes” does the patient respond about intensity, distress, frequency and interference of that symptom. Thus, although the scale appears to be very long, each patient may only need to complete the items for 8 to 14 symptoms they endorse This newly revised scale assesses each dimension on a 0 to 10 scale rather than 1-4, which increases the variance in scores and results in a more familiar and interpretable score. Correlation of CSS subscales with the Multidimensional Quality of Life Cancer scale (r = 0.34 to 0.56; P = .000) at the hypothesized levels supported construct validity. Test-retest reliability (r = 0.74-0.81) and internal consistency (alpha = .73-.74) were strong (McMillan et al., 2015).
State-Trait Anxiety Inventory (STAI)
State anxiety was assessed using the STAI (20 items) on a four-point summated rating scale (1 to 4). State scale scores can range from 20 (no anxiety) to 80 (highest anxiety). Validity of this scale has been demonstrated in various populations; in cancer patients (Gabriel et al., 2008), validity has been demonstrated by significant differences before and after getting good news about cancer (p=.02). Reliability has consistently been strong in earlier studies in cancer (Gabriel et al., 2008).
Outcome: Centers for Epidemiologic Study-Depression (CES-D)
The CES-D (Radloff, 1977) is a widely used 20-item scale that has proven useful both as a screening instrument to detect individuals at risk for depression, and to measure the symptoms of depression. Its advantage in cancer studies is that it does not include the physical symptoms (i.e. fatigue, change in appetite or weight) that are seen in cancer patients who are not depressed; thus, this scale helps to avoid over-diagnosis of depressive symptoms. The CES-D is widely used in research on depression, has been translated into multiple languages, and has impressive reliability, validity, sensitivity, and specificity (Lewinsohn et al., 1997; Irwin et al., 1999).
Appraisal of Self-Efficacy
The Cancer Behavior Inventory (Merluzzi et al., 2001) is a 12 item instrument with summated rating scales that range from 1 (not at all confident) to 9 (completely confident) designed to assess whether the patient believes that he or she can successfully enact behaviors designed to result in a desired outcome, such as improved symptom management. Higher scores indicate greater cancer self-efficacy. Evidence of validity has been shown by correlations with measures of quality of life and optimism, and negative correlations with depression and sickness impact. Cronbach alpha reliabilities ranged from .84 to .88 (Heitzmam et al., 2011).
Multidimensional Quality of Life Scale-Cancer
The Multidimensional Quality of Life Scale-Cancer (MQOL-C) will be used to assess the patients’ health-related quality of life. The MQOL-C (Pud et al., 2008; Rustoen et al., 2005) has 33 items that measure four dimensions of quality of life: physical and psychological well-being, social concerns and symptoms. Items are scaled 0 to 10 with total scale scores that may range from 0 (lowest) to 330 (highest quality); scores may be divided by the number of items to get an overall quality of life score for each patient that ranges from 0 -10. Validity was supported by correlation with measures of depression and social and physical functioning. Cronbach’s alpha is reported as .89-.91(Hoffman et al., 2009; Pud et al., 2008; Rustoen et al., 2005).
Demographic Data Form
Descriptive demographic data were collected. Patient age, gender, race/ethnicity, religious affiliation, cancer diagnosis and stage, and ECOG scores were collected from the computerized data base after patients had consented.
Data Collection Procedure
Data for this analysis came from the baseline data collected on all patients in the PCORI-funded clinical trial. Patients accrued to the clinical trial were approached in the waiting or infusion areas, asked about symptoms, and invited to participate if their symptoms met the study criteria. Patients were given the informed consent form to read, questions were answered, and if they consented, baseline data were collected at that time. Data were entered into SPSS and cleaned prior to analysis.
Data Analysis
Reliability of the study instruments were tested by using Cronbach’s Coefficient Alpha. Descriptive statistics were used to describe the demographic of patients. The Univariant Pearson correlation coefficient was obtained for each variable with QOL. Multiple regression analyses were used to derive the path coefficients: (a) the regression of anxiety on the antecedent factors, (b) the regression of depression on the antecedent factors, (c) the regression of self-efficacy on the antecedent factors, (d) the regression of QOL on the antecedent factors, (e) the regression of QOL on mediating variables, and (f) the regression of QOL on the antecedent factors and mediating variables. Hypothesis 1 states: The antecedent factors (symptom severity) will have an indirect effect on QOL of patients with cancer (i.e., their effect will be mediated through the mediating variables). This hypothesis was tested by performing the following regression analysis: (a) regression of QOL on the antecedent factors and mediating variable, (b) regression of QOL on the antecedent factors, and (c) regression of QOL on the mediating variables. Hypothesis 2 states: The mediating variables have a direct effect on QOL of patients with cancer. This Hypothesis was tested performing the following regression: (a) regression of QOL on the antecedent factors and the mediating variables, (b) regression of QOL on the antecedent factors, (c) regression of QOL on the mediating variables, (d) regression of anxiety on the antecedent factors, (e) regression of depression on the antecedent factors, and (f) regression of self-efficacy on the antecedent factors. The significance level for the standardized regression coefficients (Beta weights) was set at 0.05.
Results
Sample Characteristics
A total of 341 patients participated in the study. The participants’ ages ranged from 19 to 83 with a mean of 57.9 years (SD=12.4), and 62% were females. The most common cancer diagnoses included cancers of the breast (26.1%), lung (8.8%), and colon (7%) (Table 1). Eighty – five percent of participants were white/non-Hispanic and 52% were non-Catholic Christians (Table 1).
Table 1.
Demographic and Relevant Characteristics of Participants Characteristics
| Characteristic | Frequency | % |
|---|---|---|
| Gender | ||
| Male | 130 | 38 |
| Female | 211 | 61.9 |
| Marital Status | ||
| Married | 211 | 61.9 |
| Divorced | 59 | 17.3 |
| Single | 50 | 14.7 |
| Widow | 21 | 6.2 |
| Ethnicity | ||
| White/non-Hispanic | 287 | 85.2 |
| White/Hispanic | 14 | 4.2 |
| Black/non-Hispanic | 28 | 8.3 |
| Black/ Hispanic | 1 | 0.3 |
| Asian/Pacific | 1 | 0.3 |
| Other | 6 | 1.8 |
| Religion | ||
| Non-Catholic Christian | 177 | 52.1 |
| Catholic | 86 | 25.3 |
| Jewish | 9 | 2.6 |
| Buddhist | 1 | 0.3 |
| Other | 24 | 7.1 |
| none | 34 | 12.6 |
| Have help with health care issues | ||
| yes | 324 | 95 |
| no | 17 | 5 |
Instrument Data
The MQOL-Index total scores ranged from 82 to 310 with a mean of 211.3 (SD=42.4). The items of the index had a high internal consistency (Cronbach’s alpha (alpha = 0.89)). Scores on the CSS subscales were as follows: The symptom intensity subscale total mean score (M=81.4; SD=40.85) was relatively low on a possible scale of 0 to 330. On the same scale (0-330), symptom distress was also fairly low (M=76.4; SD=43.72). For the Frequency subscale, the mean was a little higher (M=83.8; SD= 42.5). For the Interference subscale, the mean was relatively lower (M=70.0; SD=43.15). For all subscales, alpha was higher than 0.97. Scores on the STAI (alpha =0.54) ranged from 28 to 66 with a mean of Mean of 47.03 (SD=5.7) in a possible range of 20-80. The CES-D scale scores (alpha = 0.74) ranged from 0 to 10 with a mean of 2.60 and SD=2.21. Scores on the CBI (alpha = 0.84) ranged from 40 to 203 (mean=101.59; SD=18.06.
Effect of Antecedent Factors on QOL
The theoretical model explained about 68% of the total variance in QOL (F= 180.5, P=.000). Overall, four variables accounted for the majority of the total explained variance in QOL (Table 2). These variables are anxiety, depression, self-efficacy, and symptom severity. As expected, three of them were the mediating variables. Thus, the hypothesis of that antecedent factors (symptoms severity) have an indirect influence (via mediating variables) on QOL of patients with cancer is supported.
Table 2.
Regression of Quality of Life (QOL) on the Antecedent Factors and Mediating Variables
| Variable | B | SE (B) | t | P |
|---|---|---|---|---|
| STAI | 0.605 | 0.24 | 2.542 | 0.012 |
| CES-D | -4.66 | 0.772 | -6.038 | 0 |
| CBI | 0.868 | 0.086 | 10.039 | 0 |
| Symptom Severity | -0.418 | 0.039 | -10.808 | 0 |
R2, 0.683; CBI, Cancer Behavior Inventory; CES-D, Centers for Epidemiologic Study-Depression; STAI, State-Trait Anxiety Inventory.
A second method to verify the support for Hypothesis I was to compare the results of the regression of QOL on the mediating variables (Table 3) with that of the antecedent factors. The F value for the antecedent factors regression (246.9) was less than the F value for the mediating variables (150.0), and the R2 for the antecedent factors regression (.436) was less than the R2 for the mediating variables (0.573).
Table 3.
Regression of QOL on Mediating Variables
| Variable | B | SE (B) | t | P |
|---|---|---|---|---|
| STAI | 0.395 | 0.277 | 1.427 | 0.154 |
| CES-D | -8.507 | 0.794 | -10.711 | 0 |
| CBI | 0.971 | 0.1 | 9.752 | 0 |
R2, 0.573; CBI, Cancer Behavior Inventory; CES-D, Centers for Epidemiologic Study-Depression; STAI, State-Trait Anxiety Inventory
Effect of Mediating Variables on QOL
Both the antecedent and mediating variables accounted for 68% of the total variance of QOL (Table 2). Four variables were significant in the regression equation. These variables were anxiety, depression, self-efficacy, and symptom severity. Because three of the mediating variables were significant predictors of QOL Hypothesis 2 (the mediating variables (i.e., anxiety, depression, and self-efficacy) have a direct influence on QOL of patients with cancer.) was supported. Another way to confirm the support for Hypothesis 2 was to compare the results of regression of QOL on the mediating variables (Table 3, 4) with that of regression of QOL on the antecedent factors. The comparison indicated that the F value for the mediating variables regression (150.0) was less than the F value for the antecedent factors regression (264.9), and the R2 value for the mediating variables (0.573) was higher than the R2 value for the antecedent factors regression (0.436).
Table 4.
Zero- Order Correlation of All Study Variables
| QOL total | Patient age | SS Total | STAI Total | CESD Total | CBI Total | |
|---|---|---|---|---|---|---|
| QOL Total | 1 | |||||
| Patient age | .214a | 1 | ||||
| P =0.000 | ||||||
| SS Total | -0.662a | -.130b | 1 | |||
| P =0.000 | P =0.016 | |||||
| STAI Total | 0.214a | 0.037 | -0.035 | 1 | ||
| P =0.000 | P =0.496 | P =0.523 | ||||
| CESD Total | -0.660a | -0.132b | .548a | -0134b | 1 | |
| P =0.000 | P =0.015 | P =0.000 | P =0.013 | |||
| CBI Total | 0.651a | 0.193a | -0.347a | 0.246a | -0.508a | 1 |
| P =0.000 | P =.000 | P =0.000 | P =0.000 | P =0.000 |
CBI, Cancer Behavior Inventory; CES-D, Centers for Epidemiologic Study-Depression; QOL; quality of life; STAI, State-Trait Anxiety Inventory; SS, Symptom Severity
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed).
Sub hypothesis 2-1 Anxiety Predicts QOL
We hypothesized that anxiety would be a significant predictor of QOL. This sub-hypothesis was tested by performing regression of QOL on the antecedent factors and mediating variables (Table 2). Results showed that anxiety was a significant predictor of QOL (P= 0.012), Sub-hypothesis 2-1 was supported. Another way to confirm the support for this sub hypothesis was to perform a regression of QOL on the mediating variables (Table 3); however, results showed that anxiety was not a significant predictor of QOL (P= 0.154).
Sub hypothesis 2-2 Depression Predicts QOL
We hypothesized that depression would be a significant predictor of QOL. This sub-hypothesis was tested by performing regression of QOL on the antecedent factors and mediating variables (Table 2). Results showed that depression was a significant predictor of QOL (P=0.000), therefore Sub-hypothesis 2-1 was supported. Another way to confirm the support for this sub hypothesis was to perform a regression of QOL on the mediating variables (Table 3); which also showed that depression was a significant predictor of QOL (P=0.000).
Sub hypothesis 2-3 Self-efficacy Predicts QOL
We hypothesized self-efficacy would be a significant predictor of QOL. This sub-hypothesis was tested by performing regression of QOL on the antecedent factors and mediating variables (Table 2). Results showed that self-efficacy was a significant predictor of QOL (P=.000), Sub-hypothesis 2-3 was supported. Another way to confirm the support for this sub hypothesis was to perform a regression of QOL on the mediating variables (Table 3); which also showed that self-efficacy was a significant predictor of QOL (P= 0.000).
Path Analysis Results
Path analysis is a widely used approach to studying patterns of causation among a set of variables. Path analysis, which relies on multiple linear regression, attempts to isolate the separate contributions to a dependent variable (the effect) made by a set of interrelated predictor variables (the cause) (Polit and Beck, 2010). The causal model being tested was first specified in a path diagram. As in this study, path analysis of QOL was used to test a recursive model where the causal flow is in one direction. Path analysis was used to define and explain the complex relationships in the model. This method of analysis assumes that instruments used to measure variables are reliable, that a low correlation among independent variables exists (Table 4), and that a linear relationship exists among variables. Each assumption was specifically tested in this sample before further analysis was done. Path analysis solves for the path coefficients through a series of multiple.
regression analyses
The path coefficients are the standardized regression Beta Weights (Bs) from linear regression. Once the path analysis is completed, all correlations among independent variables and dependent variables in the model can be decomposed into different effects. The decomposition consists of the total associations which are the zero-order correlations, direct effects are effects of independent variables on the dependent variable, and indirect effects are effects on the dependent variables that occur through a mediating variable. When direct and indirect effects are added together we obtain the total effect. However, not all correlations among variables are causal effects. There are two types of non-causal effects. The first is the correlations among correlated exogenous variables, which is the unanalyzed component; the second is the spurious effect which occurs with endogenous variables that are not the dependent variables.
A simplified path diagram of direct effects for all variables is shown in Figure 2. Only path coefficients significant at P ≤ .05 were drawn. Table 5 presents the total associations (Zero-Order Correlations).
Figure 2.
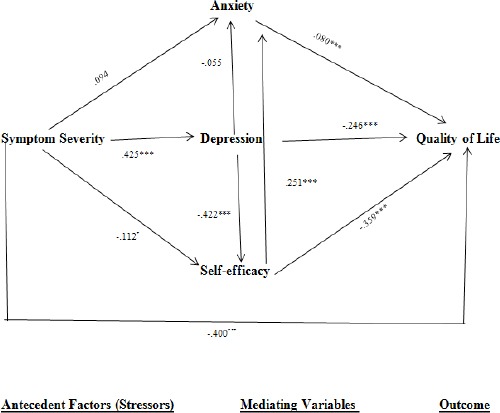
Simplified Path Diagram of Direct Effect; *p<0.05, **p<0.01, ***p<0.001
Table 5.
Decomposition of Association for Significant variables and Quality of Life (QOL)
| Variable | Type of Effect | Decomposition |
|---|---|---|
| Anxiety and QOL | Total Association | 0.214 |
| Total Effect | 0.08 | |
| Direct Effect | 0.08 | |
| Indirect Effect | NA | |
| Spurious Effect | 0.015 | |
| “(1) through Symptom Severity and self-efficacy” | 0.011 | |
| “(2) through self-efficacy, symptom severity and depression” | 0.004 | |
| Unanalyzed | 0 | |
| “(1) through age, symptom severity, self-efficacy, and depression” | 0 | |
| Depression and QOL | Total Association | -0.66 |
| Total Effect | -0.095 | |
| Direct Effect | -0.246 | |
| Indirect Effect | 0.151 | |
| (1) through self-efficacy | 0.162 | |
| “(2) through Anxiety and self-efficacy" | -0.011 | |
| “(2) through self-efficacy, symptom severity and depression” | 0.004 | |
| Spurious Effect | 0.021 | |
| “(1) through Symptom Severity, self-efficacy and Anxiety” | -0.001 | |
| “(2) through symptom severity and Self-efficacy” | 0.022 | |
| Self-efficacy and QOL | Total Association | 0.651 |
| Total Effect | -0.339 | |
| Direct Effect | -0.359 | |
| Indirect Effect | 0.02 | |
| (1) through Anxiety | ||
| Spurious Effect | 0.05 | |
| (1) through symptom severity | 0.044 | |
| “(2) through symptom severity and depression” | 0.015 | |
| “(3) through Depression and symptom severity” | -0.01 | |
| “(4) through symptom severity depression and anxiety” | 0.001 | |
| “Symptom Severity and QOL” | Total Association | -0.662 |
| Total Effect | -0.411 | |
| Direct Effect | -0.4 | |
| Indirect Effect | -0.011 | |
| (1) through depression | -0.135 | |
| (2) through self-efficacy | 0.04 | |
| (3) through self-efficacy and depression | 0.09 | |
| (4) through self-efficacy and anxiety | -0.002 | |
| “(5) through self-efficacy, anxiety and depression” | -0.004 | |
| Spurious Effect | NA |
As seen in Table 2 there were four significant variables in the regression of QOL. These variables are anxiety, depression, self-efficacy, and symptom severity. These significant variables were decomposed.
Decomposition of Significant Variables
Anxiety
Anxiety was a significant positive predictor of QOL. Table 5 presents the decomposition of association of anxiety on QOL. The zero-order relationship between these two variables was stronger than the direct effect. The major spurious effects were that of self-efficacy and symptom severity.
Depression
Depression was a significant negative predictor of QOL. Table 5 presents the decomposition of association of depression on QOL. Depression had a significant effect on QOL. The major spurious effects were that of self-efficacy and symptom severity.
Self-efficacy
The decomposition of association of self-efficacy and QOL is shown in Table 5. Self-efficacy was a significant positive predictor of QOL. The zero-order relationship between these two variables was stronger than the direct effect. Although the total effect was large, the direct effect was considerably larger than the indirect effect through anxiety. The major spurious effect was that of symptom severity.
Symptom Severity
Table 5 presents the decomposition of association of symptom severity and QOL. Symptom severity was a significant inverse predictor of QOL. The total effect was less than the zero-order relationship; it has a significant negative direct effect. However, the indirect effect was small, which strongly came from self-efficacy and depression.
Summary of Results
A multiple regression equation containing all variables accounted for 69% of the variance in QOL. Hypotheses 1 and 2 were supported. Only one antecedent factor (symptom severity) directly affected QOL at or above the established alpha level. Three mediating variables (anxiety, depression and self-efficacy) did have significant direct effect on QOL.
Path analysis of QOL revealed (a) the zero-order correlation of anxiety and QOL was larger than the direct effect, (b) the depression had a significant total effect, with the strongest direct effect, and strongest spurious effect through self-efficacy and symptom severity, (c) the zero-order correlation of self-efficacy and QOL was larger than the direct effect, and (d) symptom severity had a significant total effect, with the strongest indirect effect being through depression.
Discussion
The matrix of Pearson correlations among variables is presented in table 4. Quality of life had a significant zero-order correlation with all variables in the model. All of the mediating variables were significantly correlated with each other. However, using the criterion of .70 for theoretical and statistical problems, none of these correlations were large enough to cause concern about multicolineriaty in the regression analysis. The theoretical model explained 68% of the total variance in QOL (R2=.683, F=180.50, p<.00). None of the antecedent factors had direct effect on QOL except symptom severity, which had unexpected direct effect on QOL. Two of the mediating variables (depression and self-efficacy) were significant predictors of QOL.
The purpose of this study was to determine the relationship of antecedent factors (symptoms severity) and mediating variables (anxiety, depression, and self-efficacy) and QOL of patients with cancer.
Anxiety accounted for 5% of the explained variance. Anxiety was a significant positive predictor of QOL. The zero-order correlation was higher that the direct effect of anxiety on QOL. According to the diagram, the higher the symptom severity, the higher the anxiety. The inability of patients to exercise a sense of control over their health and activities (self-efficacy) was directly related to higher anxiety. Patient symptoms are significant stressors. Patients’ anxiety level can function as important mediators of the relationship between stressors and patient outcomes. Self-efficacy has been found to predict symptom resolution, serve as a mediator between symptom severity and functional status, and as a mediator between stress and QOL.
Depression contributed 44% of the explained variance in QOL while anxiety was not a factor in these results. Depression was a significant negative predictor of QOL in this study. This finding supports earlier research that identified physical well-being, psychological well-being, and social well-being as important dimensions of QOL (Dodge et al., 2012). However, the regression analysis demonstrated that depression had an impact on QOL in this sample, while several studies have demonstrated an association between cancer symptoms and depression (American Psychiatric Association, 2000; Laird et al., 2011; Given et al., 2004). This study suggests that depression may be one of the most important factors influencing QOL and may warrant an intervention independent of physical symptom management.
Self-efficacy contributed 42 % to the explained variance in QOL. Patients who believed strongly that they can exercise control over their health and that health was their responsibility, who attributed physical health to taking good care of themselves, and who believed that they had the power to make themselves well had higher QOL scores. The sense of exercising personal control was absent particularly from subjects with a high degree of symptom severity. In the face of greater severity of symptoms, older patients tended to experience less sense of self-efficacy, which consecutively led to lower perception of QOL. As can be seen in the path diagram, self-efficacy was significantly correlated with depression. This means that a higher perception of self-efficacy had an effect on depression (decrease depression) which led to a higher perception of QOL. Self-efficacy has been shown to reduce perceived stress and thereby improve QOL (Hoffman et al., 2009; Byma et al., 2009; Fleming et al., 2003; Merluzzi et al., 2001) Self- efficacy has been found to predict symptom resolution (Byma, Given, Given and You, 2009; Miang et al., 2008), serve as a mediator between symptom severity and functional status (Hoffman et al., 2009) and as a mediator between stress and QOL (Kreitler et al., 2007). Further it has been shown that self-efficacy is related to symptom severity (Kurtz and Given, 2008), depression (Lusczcynska et al., 2005; Mystakidou et al., 2008) and QOL (Pud et al., 2008; Merluzzi et al., 2001, Heitzmann et al., 2011).
Symptom severity was significantly related to QOL. Symptom severity contributed 44% of the variance explaining QOL. Again this is congruent with what is found in literature (Fleming et al., 2003; Motl et al., 2006; Kurtz et al., 2008; Pud et al., 2008; Merluzzi et al., 2001, Heitzmann et al., 2011). Findings indicated that the more physically disabled the patient is the more depressed the patient will be (Lusczcynska et al., 2005; Mystakidou et al., 2008). Symptom severity in the present study had a significant total effect through the indirect effect of depression. Again this supports the findings that depression was greatly affected by the symptom severity. Because of greater symptom severity, patients tended to experience more depression, less sense of self-efficacy and hence less QOL. Of special note, symptom severity had a significant zero-order correlation with QOL. This correlation suppressed the total effect of symptom severity on QOL.
The Theoretical Model
The theoretical perspective chosen for this study was the framework developed by Lazarus and Folkman (1984). It proposes that the person environmental transaction is mediated by psychosocial variables. Using this framework and the research cited, variables were selected and placed into a causal model. A multiple regression equation containing all the variables explained 69% of the variance in QOL. The theoretical framework supported the notion that the mediating variables were significant mediators of QOL. However, partial support was provided for the antecedent factors. Age affected QOL indirectly through mediating variables. Furthermore, one of the antecedent factors (symptom severity) did in fact directly affect QOL. This factor also affected QOL indirectly through the mediating variables. The findings of this study reaffirms the influence of anxiety, depression, self-efficacy, age, and symptom severity on perception of QOL in cancer patients. Based on the findings from present study, a revised causal model showing the significant variables is presented in Figure 3.
Figure 3.
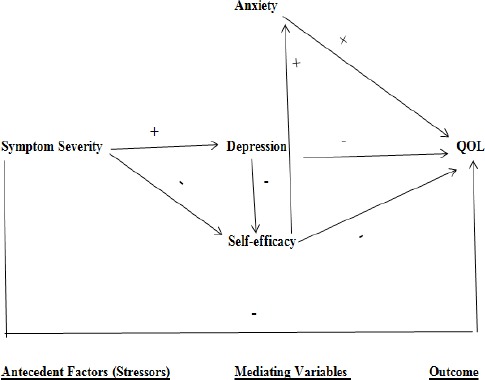
Revised Causal Model of Quality of Life
In conclusion the results of this study indicate that anxiety, depression and self-efficacy play major roles in determining the perceptions of QOL of patients who have had cancer. Nursing interventions should focus on enhancing and supporting the development of self-efficacy and managing depression. If self-efficacy is to be effective, it must assist patients to control negative psychological reactions, enhance self-efficacy, and foster return to optimal role functioning. Because age and symptom severity directly affected the mediating variables as well as symptom severity directly affecting QOL, nurses should assess these variables and consider them when identifying appropriate patient outcomes and interventions. Further research should be aimed at identifying other influential variables, with ultimate goal of developing interventions to aid patients in their efforts to manage living with cancer. Recommendation of this study includes: (1) test the revised model in similar population using a longitudinal design to evaluate the relationships among QOL over time, and (2) conduct a qualitative study that describes the process of the recovery of patients who have had cancer.
Conflict of Interest
None disclosed.
Acknowledgement
The authors express their appreciation to all participants in this study, and to Ro’ya Aldebee for data analysis.
References
- American psychiatric association. Diagnostic and statistical manual of mental disorders. Text revision (DSM-IV TR) 4th edition. Washington, DC: American Psychiatric Association press; 2000. pp. 1–20. [Google Scholar]
- Beck SL, Dudley WN, Barsevick A. Pain, sleep disturbance, and fatigue in patients with cancer:using a mediation model to test a symptom cluster. Oncol Nurs Forum. 2005;32:542–9. doi: 10.1188/04.ONF.E48-E55. [DOI] [PubMed] [Google Scholar]
- Beck SL, Towsley GL, Berry PH, et al. Core aspects of satisfaction with pain management:Cancer patients'perspectives. J Pain Symptom Managet. 2010;39:100–15. doi: 10.1016/j.jpainsymman.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy:The exercise of control. New York, NY: Academic Press; 1997. p. 2. [Google Scholar]
- Bindewald J, Oeken J, Wollbrueck D, et al. Quality of life correlates after surgery for laryngeal carcinoma. Laryngoscope. 2007;117:1770–6. doi: 10.1097/MLG.0b013e3180caa18c. [DOI] [PubMed] [Google Scholar]
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The edmonton symptom assessment system (ESAS):A simple method for the assessment of palliative care patients. J Palliat Care. 1994;7:37. [PubMed] [Google Scholar]
- Byma EA, Given BA, Given CW, You M. The effects of mastery on pain and fatigue resolution. Oncol Nurs Forum. 2009;36:544–52. doi: 10.1188/09.ONF.544-552. [DOI] [PubMed] [Google Scholar]
- Chang VT, Thaler HT, Polyak TA, et al. Quality of life and survival. Cancer. 1998;83:173–9. doi: 10.1002/(sici)1097-0142(19980701)83:1<173::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Chang VT, Hwang SS, Feuerman M, et al. Symptom and quality of life survey of medical oncology patients at a veteran affairs medical center:a role for symptom assessment. Cancer. 2000;88:1175–83. doi: 10.1002/(sici)1097-0142(20000301)88:5<1175::aid-cncr30>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients:The M.D. Anderson symptom inventory. Cancer. 2000;89:1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Cunningham A, Lockwood G, Cunningham J. A relationship between perceived self-efficacy and quality of life in cancer patients. Patient Educ Couns. 1991;17:71–8. doi: 10.1016/0738-3991(91)90052-7. [DOI] [PubMed] [Google Scholar]
- De Aguiar SS, Bergmann A, Mattos IE. Quality of life as a predictor of overall survival after breast cancer treatment. Qual Life Res. 2014;23:627–37. doi: 10.1007/s11136-013-0476-8. [DOI] [PubMed] [Google Scholar]
- de Graff A, de Leeuw JR, Ros WJ, et al. Pretreatment factors predicting quality of life after treatment for head and neck cancer. Head Neck. 2000;22:398–407. doi: 10.1002/1097-0347(200007)22:4<398::aid-hed14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Donnelly S, Walsh D, Rybicki L. The symptoms of advanced cancer:identification of clinical and research priorities by assessment of prevalence and severity. Palliative Care. 1995;11:27–32. [PubMed] [Google Scholar]
- Dodge R, Annette P, Daly AP, Huyton J, Sanders LD. The challenge of defining wellbeing. Int J Wellbeing. 2012;2:222–35. [Google Scholar]
- Finlay IG, Dunlop R. Quality of life assessment in palliative care. Ann Oncol. 1994;5:13–8. doi: 10.1093/oxfordjournals.annonc.a058677. [DOI] [PubMed] [Google Scholar]
- Fleming G, McKenna M, Murchison V, et al. Using self-efficacy as a client-centered outcome measure. Nurs Stand. 2003;17:33–36. doi: 10.7748/ns2003.05.17.34.33.c3386. [DOI] [PubMed] [Google Scholar]
- Gabriel GS, Lah M, Barton M, et al. Do cancer follow-up consultations create anxiety? J Psychosoc Oncol. 2008;26:17–30. doi: 10.1300/j077v26n01_02. [DOI] [PubMed] [Google Scholar]
- Gil F, Costa G, Hilker I, Benito L. First anxiety, afterwards depression:psychological distress in cancer patients at diagnosis and after medical treatment. Stress Health. 2012;28:362–7. doi: 10.1002/smi.2445. [DOI] [PubMed] [Google Scholar]
- Given C, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol. 2004;22:507–16. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- Given B, Given CW, Sikorskii A, et al. Establishing mild, moderate, and severe scores for cancer-related symptoms:How consistent and clinically meaningful are interference-based severity cut-points? J Pain Symptom Manage. 2008;35:126–35. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzmann CA, Merluzzi TV, Jean-Pierre P, et al. Assessing self-efficacy for coping with cancer:Development and psychometric analysis of the brief version of the cancer behaviorinventory (CBI-B) Psychooncology. 2011;20:302–12. doi: 10.1002/pon.1735. [DOI] [PubMed] [Google Scholar]
- Hoffman AJ, Von Eye A, Gift GA, et al. Testing a theoretical model of perceived self-efficacy for cancer-related fatigue self-management and optimal physical functional status. Nurs Res. 2009;53:32–41. doi: 10.1097/NNR.0b013e3181903d7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood P, Stephens R-J. Depression in patients with lung cancer;prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;4:893–906. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- Irwin M, Artin KH, Oxmnan MN. Screening for depression in the older adult:Criterion validity of the 10-item center for epidemiological studies depression scale. Arch Intern Med. 1999;159:1701–4. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- Jim H, Andrykowski M, Munster P, Jacobsen P. Physical symptoms/side effects during breast cancer treatment predict posttreatment distress. Ann Behav Med. 2008;34:200–8. doi: 10.1007/BF02872674. [DOI] [PubMed] [Google Scholar]
- Kreitler S, Peleg D, Ehrenfeld M. Stress, self-efficacy and quality of life in cancer patients. Psychooncology. 2007;16:329–41. doi: 10.1002/pon.1063. [DOI] [PubMed] [Google Scholar]
- Kurtz MI, Kurtz JC, Given CW, Given BA. Patient optimism and mastery –do they play a role in cancer patients'management of pain and fatigue? J Pain Symptom Manage. 2008;36:1–10. doi: 10.1016/j.jpainsymman.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Kurtz ME, Kurtz JC, Stommel M, Given CW, Given B. Physical functioning and depression among older persons with cancer. Cancer Practice. 2001;9:11–8. doi: 10.1046/j.1523-5394.2001.91004.x. [DOI] [PubMed] [Google Scholar]
- Landers M, Savage E, McCarthy G, Fitzpatrick JJ. Self-care strategies for the management of bowel syndrome following sphincter-saving surgery for rectal cancer. Clin Oncol Nurs. 2011;15:105–13. doi: 10.1188/11.CJON.E105-E113. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. pp. 22–70. [Google Scholar]
- Laird BJA, Scott AC, Colvin LA, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage. 2011;42:1–11. doi: 10.1016/j.jpainsymman.2010.10.261. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for epidemiological studies-depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–87. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- Liang S-Y, Yates P, Edwards H, Tsay SL. Development and initial evaluation of reliability and validity of the opioid-taking self-efficacy scale. Oncol Nurs Forum. 2008;35:62–9. [Google Scholar]
- Linden W, Girgis A. Psychological treatment outcomes for cancer patients:What do meta-analyses tell us about distress? Psychooncology. 2012;21:343–350. doi: 10.1002/pon.2035. [DOI] [PubMed] [Google Scholar]
- Li TC, Li CI, Tseng CH, et al. Quality of life predicts survival in patients with non-small cell lung cancer. BMC Public Health. 2012;12:790. doi: 10.1186/1471-2458-12-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusczcynska A, Gutierrez-Dona B, Schwarzer R. General self-efficacy in various domains of human functioning:Evidence from five countries. Int J Psychol. 2005;40:80–9. [Google Scholar]
- Maciejewski P, Zhang B, Block S, Prigerson H. An empirical examination of the stage theory of grief. JAMA. 2007;297:716–23. doi: 10.1001/jama.297.7.716. [DOI] [PubMed] [Google Scholar]
- McMillan SC, Tofthagen C, Choe R, Rheingans J. Assessing symptoms experienced by patients with cancer:occurrence, intensity, distress, interference and frequency. J Hosp Palliat Nurs. 2015;17:56–65. [Google Scholar]
- McMillan SC, Small BJ. Symptom distress and quality of life in hospice patients with cancer. Oncol Nurs Forum. 2002;29:1421–8. doi: 10.1188/02.ONF.1421-1428. [DOI] [PubMed] [Google Scholar]
- Merluzzi TV, Nairn RC, Hegde K, Martinez Sanchez MA, Dunn L. Self-efficacy for coping with cancer:revision of the cancer behavior inventory (Version 2.0) Psychooncology. 2001;10:206–17. doi: 10.1002/pon.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Dodd M, West C, et al. The use of a responder analysis to identify differences in patient outcomes following a self-care intervention to improve cancer pain management. Pain. 2007;129:55–63. doi: 10.1016/j.pain.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl RW, Snook EM, McAuley E, Gliottoni RC. Symptoms, self-efficacy, and physical activity among individuals with multiple sclerosis. Res Nurs Health. 2006;29:597–606. doi: 10.1002/nur.20161. [DOI] [PubMed] [Google Scholar]
- Monroe B, Oliviere D. Resilience in palliative care. Eur J Palliat Care. 2006;13:22–5. [Google Scholar]
- Mount B. Whole person care:beyond psychosocial and physical needs. Am J Hosp Palliat Care. 1993;10:28–37. doi: 10.1177/104990919301000109. [DOI] [PubMed] [Google Scholar]
- Murphy BA, Rilder S, Wills N, Dietrich M. Quality of life research in head and neck cancer:a review of the current state of the science. Crit Rev Oncol Hemat. 2007;62:251–67. doi: 10.1016/j.critrevonc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Mystakidou K, Parpa E, Tsilika E, Galanos A, Vlahos L. General perceived self-efficacy:validation analysis in Greek cancer patients. Support Care Cancer. 2008;16:1317–22. doi: 10.1007/s00520-008-0443-z. [DOI] [PubMed] [Google Scholar]
- Naughton M, Hansi J. Symptom assessment in cancer patients. Curr Oncol Rep. 2007;4:256–63. doi: 10.1007/s11912-002-0024-0. [DOI] [PubMed] [Google Scholar]
- Omran S, Saeed AM, Simpson J. Symptom distress of Jordanian patients with cancer receiving chemotherapy. Int J Nurs Pract. 2012;18:125–32. doi: 10.1111/j.1440-172X.2012.02012.x. [DOI] [PubMed] [Google Scholar]
- Peplau HE. Quality of life:An interpersonal perspective. Nurs Sci Q. 1994;7:10–4. doi: 10.1177/089431849400700107. [DOI] [PubMed] [Google Scholar]
- Polit DF, Beck CT. Essentials of nursing research:evidence for nursing practice. Philadelphia: Wolters Kluwer Health/ Lippincott Williams and Wilkins; 2010. pp. 427–563. [Google Scholar]
- Portenoy RK, Thaler HT, Kornblith AB, et al. The memorial symptom assessment scale:An instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30:1326–36. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Pud D, Ben Ami S, Cooper BA, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage. 2008;35:162–70. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale:A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rustoen T, Moum T, Padilla G, Paul S, Miasowski C. Predictors of quality of life in oncology Outpatients with Pain from Bone Metastasis. J Pain Symptom Manage. 2005;30:234–42. doi: 10.1016/j.jpainsymman.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Soltow D, Given BA, Given CW. Relationship between age and symptoms of pain and fatigue in adults undergoing treatment for cancer. Cancer Nurs. 2010;33:296–303. doi: 10.1097/NCC.0b013e3181ce5a1a. [DOI] [PubMed] [Google Scholar]
- Thompson SC, Sobolew-Shubin A, et al. Maintaining perceptions of control:finding perceived control in low-control circumstances. J Pers Soc Psychol. 1993;64:293–304. doi: 10.1037//0022-3514.64.2.293. [DOI] [PubMed] [Google Scholar]
- Tofthagen C, McMillan SC. Pain, neuropathic symptoms and physical and mental well-being in persons with cancer. Cancer Nurs. 2010;33:1–8. doi: 10.1097/NCC.0b013e3181e212b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammer J-E, Heyland D, Dudgeon D, et al. Measuring the symptom experience of seriously ill cancer and non-cancer hospitalized Patients near the end of life with the Memorial Symptom Assessment Scale. J Pain Symptom Managet. 2003;25:420–9. doi: 10.1016/s0885-3924(03)00074-5. [DOI] [PubMed] [Google Scholar]
- Williams P, Ducey K, Sears A, et al. Treatment type and symptom severity among oncology patients by self-report. Int J Nurs Studies. 2001;38:359–67. doi: 10.1016/s0020-7489(00)00067-5. [DOI] [PubMed] [Google Scholar]


