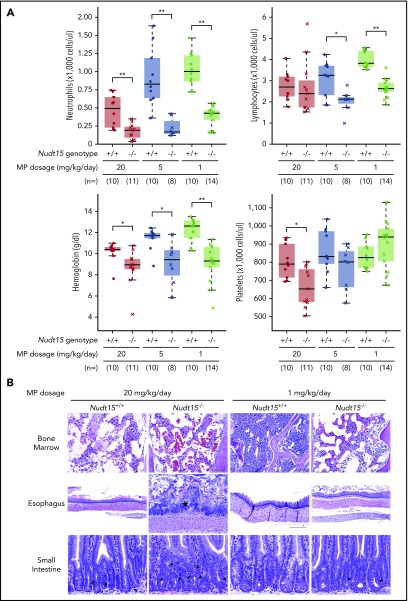
Figure 3.
Thiopurine-induced hematopoietic and gastrointestinal toxicity in Nudt15−/−and wild-type mice. (A) Hematological parameters were measured after 7 days of MP therapy (20, 5, or 1 mg/kg per day, shown in red, brown, and green, respectively) by complete peripheral blood count in Nudt15−/− and wild-type animals (crosses and dots, respectively). (B) Thiopurine-induced damage to the bone marrow, esophagus, and small intestine was examined at the time of death resulting from toxicity. Wild-type mice receiving 1 mg/kg per day showed no sign of morbidity from thiopurine treatment and were euthanized on day 26. Bone marrow cellularity was normal in wild-type mice receiving 1 mg/kg per day, whereas in all other conditions, loss of bone marrow was evident by increased empty spaces (upper panel). In the esophagus, an area of severe mucosal ulceration is shown in the Nudt15−/− mice receiving 20 mg/kg per day; the starred area indicates a mat of bacteria overlying the ulcer (middle panel). Black arrowheads point to apoptotic crypt epithelial cells in the small intestine (lower panel). Hematoxylin and eosin staining; scale bars: upper panel, 100 µm; middle panel, 250 µm; lower panel, 50 µm. *P < .05, **P < .01, as estimated using Wilcoxon rank test.