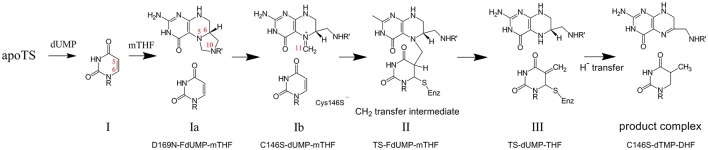
Figure 1.
Chemical mechanism of thymidylate synthase, as determined from kinetic studies on the E. coli enzyme. Atom numbering is shown in red numbers. The numbering of each intermediate is in accordance with Stroud and Finer-Moore (2003), and the specific ecTS variants and ligands used to mimic those intermediates are indicated. For step Ib, “Cys146S−” indicates the location of residue 146 (E. coli TS numbering) that is mutated to serine to trap at step Ib and that in the wild-type enzyme is the active site nucleophile that bonds to C6 of dUMP.