Figure 4.
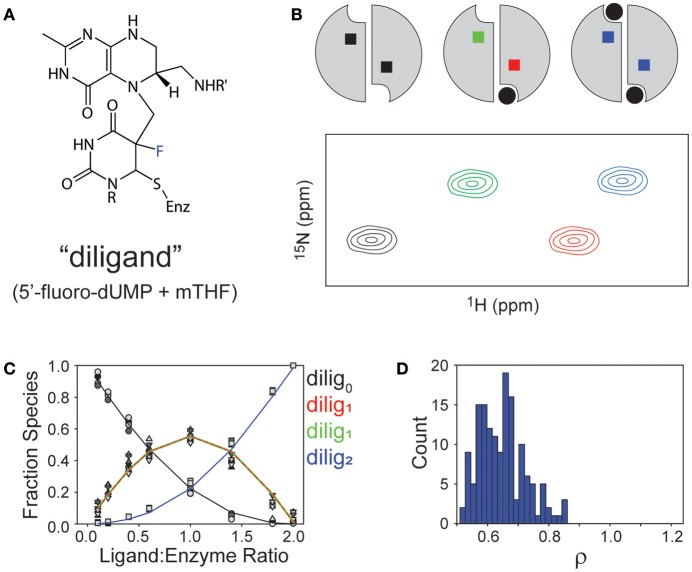
Observations of binding “diligand” to ecTS. (A) Chemical structure of stable “diligand” as a model for intermediate II in the reaction mechanism (see Figure 1). The fluorine (blue) is a poor leaving group, which stabilizes this intermediate. (B) Schematic of the three liganded states of the TS homodimer (apo, lig1, lig2) and a resultant “peak quartet.” Peaks are color coded to correspond with the liganded state (black = apo, blue = saturated) and to discriminate bound (red) and empty (green) subunits of the lig1 state. (C) Fitting of relative Kd values from quartet peak intensities, as reported in Sapienza et al. (ref 29). (D) Range of ρ values from monte carlo simulations of fit in (C).