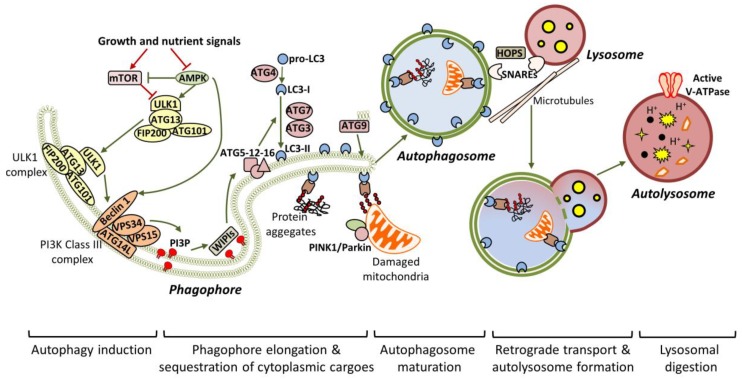
Figure 1.
General autophagic mechanisms. Under nutrient-rich conditions, the autophagic pathway remains inactive due to mTOR-dependent phosphorylation of ULK1. Nutrient deprivation or stress, activate AMPK, which promotes autophagy initiation, through mTOR inhibition and concurrent ULK1 activation. Once active, the ULK1 complex is translocated to the pre-autophagosomal membrane structures (mainly derived by the endoplasmic reticulum (ER) or endosomal membranes), to initiate the formation of the phagophore. ULK1- and AMPK-dependent phosphorylation of Beclin 1 disrupt its interaction with anti-autophagic factors (like Bcl-2) and enhance the recruitment of the VPS34 complex. Subsequently, the active VPS34 complex acts as a PI3K to generate PI3P. The resulted PI3P interacts with WIPI proteins, which in turn promote the recruitment of autophagy-related gene (ATG) proteins, required for phagophore nucleation. The critical step for autophagosome formation is the conjugation of LC3/ATG8 to PE lipids, a reaction that requires the co-ordinated function of ATG7, ATG3 and ATG5-12-16 (acting as E1, E2 and E3 enzymes respectively). In parallel, ATG9 promotes autophagosome formation, as it provides lipids to the expanding phagophore. During expansion, specific adaptors sequester cytoplasmic material (like protein aggregates or damaged mitochondria) in the phagophore, by directly interacting with LC3-II. Scission events mediate the closure of the phagophore and the formation of the double-bilayered autophagosome. The newly-formed autophagosomes enter the microtubule-dependent retrograde transport to attach lysosomes. Autophagosome-lysosome attachment is aided by multiple proteins, including soluble NSF attachment protein receptors (SNAREs) and the scaffolding homotypic fusion and protein sorting (HOPS) complex. Following attachment, the outer autophagosomal membrane fuses with the single lysosomal membrane, resulting in the autolysosome. Efficient acidification (mediated by v-ATPase) activates the lysosomal hydrolases, which finally digest the sequestered cytoplasmic material.