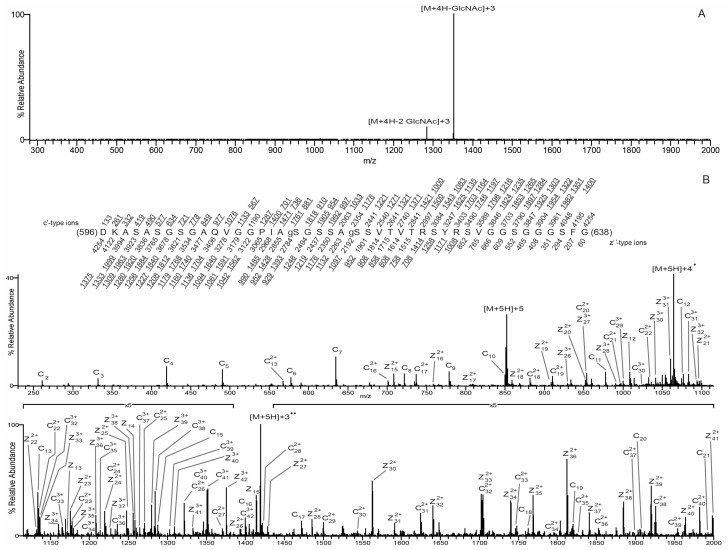
Figure 5.
O-GlcNAc site mapping on the S612A/T643A-mutated mature lamin A tail via liquid chromatography-MS/MS. (A) CAD MS/MS spectrum recorded on the (M + 4H)4+ ions (m/z 1064.2561) of the di-GlcNAcylated peptide DKASASGSGAQVGGPIAgSGSSAgSSVTVTRSYRSVGGSGGGFG. The CAD spectrum contains the charge-reduced ions minus the loss of 1GlcNAc, 203 Da, at m/z 1351, and the charge-reduced ion minus the loss of 2 GlcNAc residues, 406 Da, at m/z 1284; (B) an ETD MS/MS spectrum recorded on the [M + 5H]5+ ions (m/z 852.0084) of the di-GlcNAcylated peptide DKASASGSGAQVGGPIAgSGSSAgSSVTVTRSYRSVGGSGGGFG. Predicted product ions of types c’- and z’•- are listed above and below the peptide sequence, respectively. Singly charged ions are listed as monoisotopic masses; doubly and triply charged ions are listed as average masses. ETD product ions are labeled in the ETD spectrum. Observed product ions are underlined and are sufficient to define the O-GlcNAc residues at Ser612 and Ser618, indicated by ‘gS’ in the amino acid sequence.