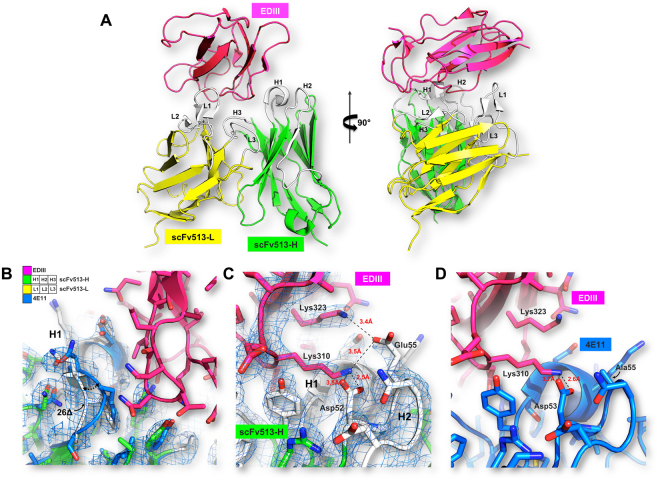
Figure 2.
Interactions between scFv513 and DIII from DENV4 derived from crystallography. (A) Overall views at two angles separated by 90° of the complex between scFv513 (VH and VL depicted as green and yellow ribbons respectively) and the domain III of the envelope protein from DENV4 (red ribbons). Coordinates were taken from molecule A of PDB code 5AAM. CDRs and N-and C-termini are labeled. (B) Details of the interactions and comparison with 4E11: panels (C) and (D) show a key contact region where an additional salt bridge was introduced in scFv513 between Glu55H2, (Kabat numbering) and Lys323 (EDIII), which is absent in the complex between scFvE11 and EDIII (panel D). This results from the Ala to Glu substitution in the H2 region of 4E11 (see text). The S26Δ deletion introduced in VH of 513 (white tube) removing the bulge present in 4E11 (blue tube) is displayed in (B) and the movement between the two polypeptide chains is shown by an arrow. The electron density map (coefficients 2Fo-Fc at a level of 1σ) is overlaid in panels (B) and (C).