Figure 4.
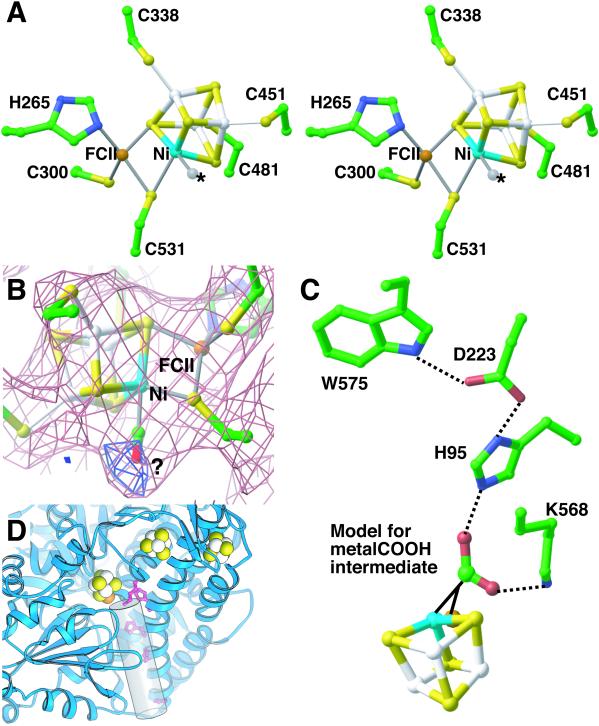
(A) Stereoview of the C-cluster. The identity of the ligand to Ni (Ma site) is unknown (position marked with an *). Same color scheme as Fig. 3. (B) A 2.8-Å resolution (2Fobs − Fcalc) electron density map (pink) for the C-cluster. The electron density is spread out near Cys531, and the exact position of the side chain is difficult to determine. Positive difference (Fobs − Fcalc) electron density maps (blue) suggest the presence of an additional ligand to Ni. (C) Model of metal–COOH intermediate. If COOH is placed in the general area of the open coordination sites on Ni and FCII, it can interact with His95 and Lys568. His95, which is part of a hydrogen-bonding network with Asp223 and Trp575 and is ≈2.6 Å away from the modeled intermediate, could act as the catalytic base. Lys568, which is within hydrogen-bonding distance (≈2.5 Å), could stabilize the intermediate. (D) Putative tunnel (shown as a cylinder), identified by visual inspection of electron density maps, leading from the active site to solvent in R. rubrum CODH. His95 and Lys568 (red ball-and-stick) sit at the top of the tunnel. Histidine residues 98, 101, and 108 (red ball-and-stick), conserved among CODH sequences, line the putative tunnel.