Table 1. Optimization of the reaction conditions a .
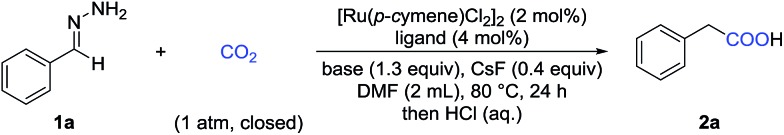
| |||
| Entry | Ligand | Base | Yield b (%) |
| 1 | dppp | Cs2CO3 | 44 |
| 2 | dppe | Cs2CO3 | 19 |
| 3 | dppf | Cs2CO3 | 85 (83) |
| 4 | dppf | K3PO4 | 51 |
| 5 | dppf | KOtBu | 80 |
| 6 | dppf | DBU | 27 |
| 7 c | dppf | Cs2CO3 | 73 |
| 8 d | dppf | Cs2CO3 | 72 |
| 9 | No | Cs2CO3 | 13 |
| 10 | dppf | No | 31 |
| 11 e | dppf | Cs2CO3 | 78 |
| 12 f | dppf | Cs2CO3 | N.D. |
| 13 g | dppf | Cs2CO3 | N.D. |
aReaction conditions: 1a (0.4 mmol).
bYields were determined by crude 1H NMR using dibromomethane as an internal standard, and the isolated yields are given in parentheses.
cBase (0.4 mmol).
dBase (0.6 mmol).
eNo CsF.
fNo [Ru(p-cymene)Cl2]2.
gN2 instead of CO2. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene. N.D. = not detected.
