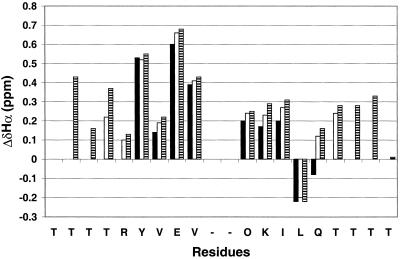
Figure 3.
ΔδαH = observed δαH − random coil δαH for the strand residues DP (filled bars), DP-TT (open bars) and DP-TT2 (striped bars), 1 mM each in aqueous (9:1 H2O/D2O) sodium deuteroacetate buffer, pH 3.8 (uncorrected), 4°C. The reported random coil δαH value for lysine was used for ornithine (random coil δαH values from ref. 47). No data are shown for the N-terminal residue of each peptide because this terminus is uncapped. For DP-TT2, δαH of Thr-17 and Thr-18 could not be unambiguously assigned; the ΔδαH values shown for these two residues are based on the average of the two observed δαH values (4.54 and 4.72 ppm). Chemical shifts were externally referenced to 2,2-dimethyl-2-silapentane-5-sulfonate (DSS).