Figure 9.
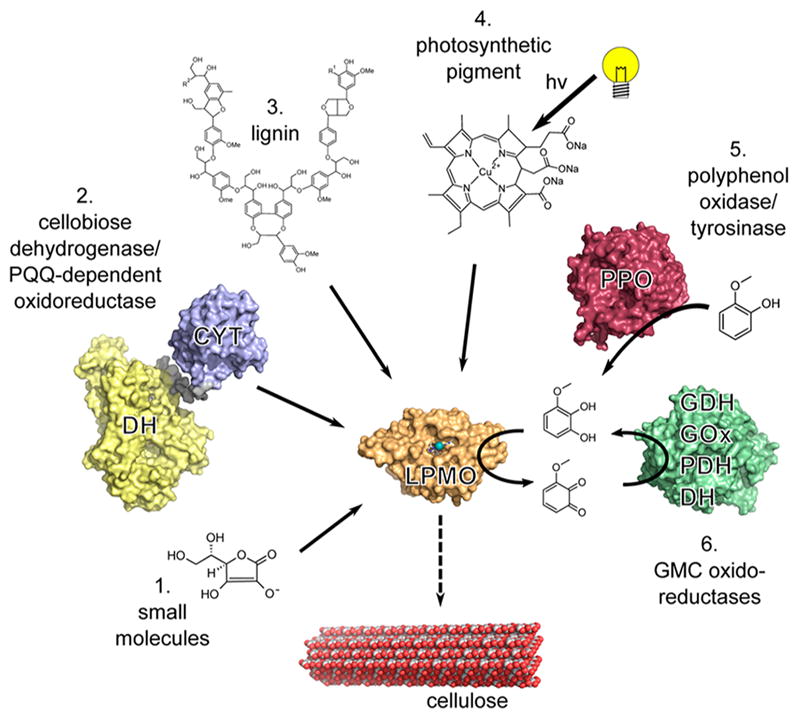
Examples of electron sources that can reduce the LPMO (orange) active-site copper (teal). (1) Small molecules such as ascorbate can donate electrons directly.3 (2) Enzymatic electron donors such as cellobiose dehydrogenase (CDH) can transfer electrons from the catalytic dehydrogenase domain (DH, yellow) via the cytochrome heme domain (CYT, blue) to a LPMO.145 Pyrroloquinoline quinone (PQQ)-dependent pyranose dehydrogenase has been postulated to transfer electrons in a similar fashion.146 (3) Insoluble high-molecular-weight lignin can serve as a reservoir for electrons facilitating LPMO activity.6 (4) Excited photosynthetic pigments can provide electrons for LPMO activity.147 (5) Polyphenol oxidase (red) can generate small molecule electron donors for LPMO activity from lignin building blocks.148 (6) Principle of regeneration of an oxidized quinoid form of electron donor by glucose-methanolcholine (PMC) oxidoreductases (green) such as glucose dehydrogenase (GDH), glucose oxidase (GOx), pyranose dehydrogenase (PDH), and the dehydrogenase domain of CDH (DH).4 Adapted with permission from ref 4. Copyright 2016 The American Association for the Advancement of Science.
