Abstract
Incarcerated populations experience elevated burdens of infectious diseases, which are exacerbated by limited access to prevention measures. Dynamic models are used to assess the spread and control of diseases within correctional facilities and repercussions on the general population. Our systematic review of dynamic models of infectious diseases within correctional settings identified 34 studies published between 1996 and 2017. Of these, 23 focused on disease dynamics and intervention in prison without accounting for subsequent spread to the community. The main diseases modeled in these studies were human immunodeficiency virus (HIV; n = 14, 41%), tuberculosis (TB; n = 10, 29%), and hepatitis C virus (HCV; n = 7, 21%). Models were fitted to epidemiologic data in 14 studies; uncertainty and sensitivity analyses were conducted in 8, and validation of model projection against empirical data was done in 1 study. According to the models, prison-based screening and treatment may be highly effective strategies for reducing the burden of HIV, TB, HCV, and other sexually transmissible infections among prisoners and the general community. Decreasing incarceration rates were projected to reduce HIV and HCV infections among people who inject drugs and TB infections among all prisoners. Limitations of the modeling studies and opportunities for using dynamic models to develop quantitative evidence for informing prison infection control measures are discussed.
Keywords: correctional facilities, infectious diseases, mathematical modeling, transmission dynamics
INTRODUCTION
More than 10 million individuals are incarcerated globally (1). In the United States, 1% of the adult population is incarcerated, the highest rate in the world, and more than 9% are incarcerated at some point in their lifetime (2, 3). Compared with the general population, prisoners and detainees worldwide experience a higher burden of infectious diseases, such as human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (4), viral hepatitis (4), tuberculosis (TB) (4), and a range of sexually transmissible infections (5, 6). This high burden of infectious diseases in correctional facilities is driven not only by the higher prevalence of infection among incoming prisoners (7) but also by contextual factors within prisons that contribute to a higher risk of disease transmission among prisoners. Such factors include risk behavior, overcrowding, delay or lack of diagnosis and treatment, limited access to clean water, inadequate sanitation, and lack of harm-reduction measures such as condoms, sterile tattooing equipment and syringes, and drug treatment (6, 8, 9). Compounding these risk factors, criminalization of drug use and imprisonment of people who use drugs have resulted in a repetitive cycle of incarceration of many individuals infected with HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), and TB, and those at high risk of infection, such as people who inject drugs (PWID) (4, 10–12). This disproportionate burden and risk of infection within correctional facilities has been hypothesized to be fueling epidemics of HIV, viral hepatitis, and TB in the general population through regular cycling of infected, at-risk individuals in and out of incarceration (13).
Mathematical modeling is a method of simulating epidemiologic systems that uses mathematical concepts to identify those systems’ driving factors and forecast their future behavior. When empirical data are available to inform model structure, parameterization, and validation, mathematical modeling can be an effective tool to understand trends and patterns of diseases dynamics and to evaluate the effectiveness of public health intervention measures and innovative technologies (14, 15). Several models have been developed to analyze the dynamics of infectious diseases within correctional settings and their contribution to disease transmission within the general population. Some of these models also are used to evaluate the effectiveness and cost-effectiveness of prison-based health intervention measures such as screening and treatment, condom distribution, and educational programs. Approaches to mathematical modeling of infectious disease may be static or dynamic (16). Static models only account for the direct health effect of disease control intervention on individuals by assuming they are subject to a constant risk of disease exposure unaffected by the intervention (17, 18). Dynamic models are more suitable for infectious disease modeling because they account for disease transmission dynamics and capture the direct and indirect effects of intervention measures (17, 18). We conducted a systematic review of dynamic transmission models for infectious diseases in prisons. We also examined the modeling approaches and techniques used to develop these prison-based disease transmission models and generate relevant health outcomes. Here, we assess model structures and underlying assumptions relative to the potential contribution of incarceration to disease transmission inside and outside correctional facilities, and discuss evidence provided by dynamic models on transmission dynamics and control of prison-based epidemics. We highlight the insights provided by these models on the interaction among incarceration, disease dynamics, and public health intervention measures.
METHODS
To systematically review the literature, 7 databases—Academic Search Premier, Criminal Justice Abstracts, EMBASE, MathSciNet, PubMed, ScienceDirect, and Web of Science—were searched using predefined keywords (Web Tables 1 and 2, available at https://academic.oup.com/aje). The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for articles published between January 1, 1970, and March 10, 2017. In addition, reference lists of included studies were reviewed, but these did not yield any additional articles. Our review focused on the type of dynamic models used in modeling prison-based infectious diseases dynamics, the diseases studied, populations and settings of interest, and public health interventions. We highlighted evidence provided by mathematical models on disease dynamics and control within and between correctional facilities and surrounding local communities.
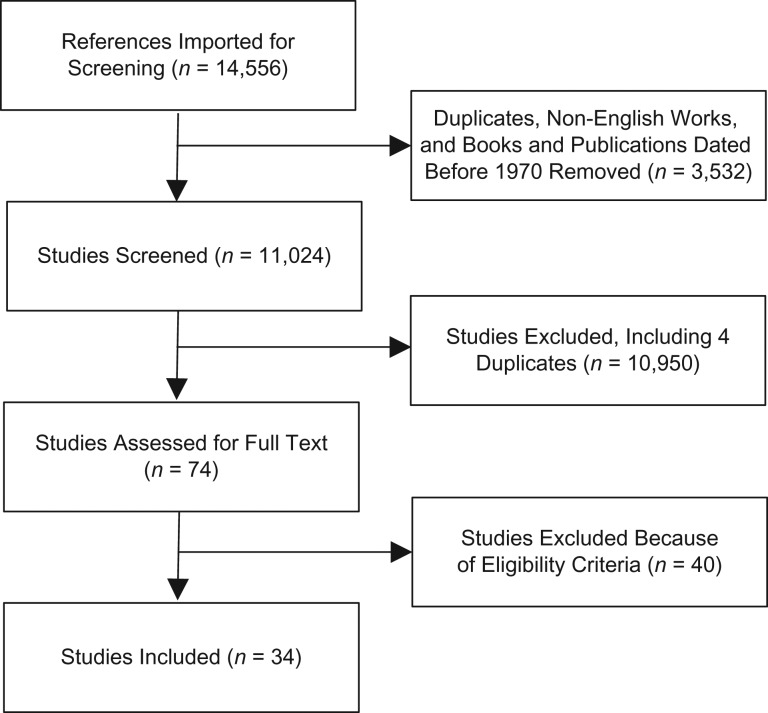
Journal articles were eligible for inclusion if they met the following criteria: 1) published after 1970; 2) written in English; 3) peer-reviewed; 4) focused on an infectious disease within a correctional facility setting; and 5) described a mathematical model that accounted for disease transmission dynamics. Editorials and books were excluded. Two authors independently reviewed the title and abstract of each article identified through the database search; a third author was involved as needed to reach consensus (Figure 1). This protocol was repeated for each full-text article accepted during the initial screening (Web Table 3). To ensure consistency, each of these authors also extracted data from the included articles. The extracted information included publication date; study objective; disease focus; disease transmission routes; targeted populations and settings; intervention approach; model outcome measures; risk behavior; type of model; model fitting procedure; empirical data; uncertainty analysis; and sensitivity analysis (Web Table 4).
Figure 1.
Flow chart of the review process and criteria for article inclusion. Studies were excluded if they were duplicates, not peer reviewed, did not include consideration of an infectious disease within an incarceration setting, or did not describe a mathematical model that accounted for the dynamics of disease transmission. For studies to be eligible for inclusion, researchers had to have 1) considered an incarceration setting, 2) assessed the transmission dynamics of an infectious disease, and 3) used a dynamic transmission model.
RESULTS
Through our systematic review process, we identified 34 eligible articles published between 1996 and 2017. In most of the studies, transmission dynamics within incarcerated populations from specific geographical settings were investigated, most frequently the United States (n = 10, 29%), Brazil (n = 5, 15%), United Kingdom (n = 4, 12%), Australia (n = 2, 6%), Eastern Europe (n = 3, 9%), and Africa (n = 3, 9%), whereas in 8 (23%) studies, generic incarceration settings were modeled. Transmission within a single prison was modeled in most studies (n = 19, 56%), the interplay between prison-based and community-based epidemics (n = 11, 32%) was considered in some, and a network of prisons was assessed in 4 (12%).
High rates of reincarceration and within-prison movements are fundamental to disease transmission dynamics within correctional facilities and the general population. In prison-community models, incarceration dynamics were parameterized from rates of first-time incarceration and reincarceration, and the duration of incarceration. These parameters were assumed to be fixed over time, with some models accounting for age-stratified variation (19–22), injection drug–use status (PWID vs. non-PWID) (19, 20), and duration of injection drug use (23). For each model, the parameters were specific to the country of interest, with the exception of a generic model that used global averages for parameters values (4).
Two classes of model were reviewed: compartmental models (n = 33, 97%) and an agent-based model (n = 1, 3%). The agent-based model tracked 2 million individuals representing the US population, each with their own characteristic traits such as sex, age, drug-infection behavior (i.e., active or former PWID vs. non-PWID), imprisonment status (i.e., incarcerated in prisons vs. general population), contact network, and disease status (20). The model accounted for the importance of individual-level interactions and how an individual’s traits change over time (20). In compartmental models, individuals were aggregated into compartments according to disease status, age (13, 19, 21–23), drug-injection behavior (i.e., non-PWID, active PWID, and former PWID) (4, 13, 19, 21–23), syringe-sharing behavior (4), and incarceration status (i.e., never incarcerated, currently incarcerated, recently incarcerated, and previously incarcerated) (4, 13, 19, 21–23). Contrary to agent-based models, compartmental models have a clearer relationship between parameters and observed outcomes; hence, they are easier to parameterize and fit to data.
The infectious diseases modeled included HIV, TB, HBV, HCV, chlamydia, gonorrhea, syphilis, and methicillin-resistant Staphylococcus aureus (MRSA) (Table 1). In most of the studies (n = 23, 68%), the effectiveness and cost-effectiveness of prison-based intervention measures, such as screening and treatment, condom distribution, opioid agonist therapy (OAT), and HBV vaccination, were measured (Table 2). In 7 studies (21%), researchers analyzed the long-term dynamics of disease outbreak within correctional facilities; in 4 studies (12%), the authors developed theoretical frameworks to assess the risks of infection in prison.
Table 1.
Articles Published 1996–2017 and Reviewed for Each Infectious Disease (n = 34)a
| Disease | No. of Articles | % | References |
|---|---|---|---|
| HIV | 14 | 41 | 4, 13, 25, 29, 32, 33, 39–46 |
| TB | 10 | 29 | 25, 31, 34, 35, 45, 51–53, 65, 66 |
| HCV | 7 | 21 | 20–23, 42, 56, 57 |
| HBV | 2 | 6 | 19, 58 |
| Chlamydia | 4 | 12 | 29, 30, 58, 59 |
| Syphilis | 3 | 9 | 29, 42, 58 |
| Gonorrhea | 3 | 9 | 29, 30, 58 |
| MRSA | 2 | 6 | 26, 60 |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MRSA, methicillin-resistant Staphylococcus aureus; TB, tuberculosis.
a Some of these articles cover multiple diseases.
Table 2.
Summary of Mathematical Models Used to Evaluate the Effect of Prison-Based Strategies on the Control of Infectious Diseases in 34 Studies, 1996–2017
| First Author, Year (Reference No.) | Model Type | Strategies Evaluated | Prevalence/Risk Without Intervention | Intervention Effectiveness/Cost-Effectiveness | Model Limitations | |||
|---|---|---|---|---|---|---|---|---|
| Prison/Jail | Community | |||||||
| Dolan, 2016 (4) | Deterministic compartmental model of HIV transmission among PWID inside and outside of prison | Prison-based OAT followed by postrelease retention, reduction of incarceration rate among PWID | HIV prevalence of 5.7%–28.6% for incarcerated PWID in medium-risk community, and 22%–80% prevalence in high-risk community | HIV prevalence of 5%–20% among PWID for medium-risk community, and prevalence >20% among PWID for high-risk community |
|
|
||
| Tuli, 2009 (29) | Deterministic compartmental model of HIV transmission among MSM inside and outside of jail | Screening, treatment, and condom provision | HIV prevalence 13.4% (also considered 6% and 25% prevalence) | HIV prevalence 6.7% | Intervention was cost-saving for averting HIV infections in the United States, if at least 20% of screened inmates used condoms |
|
||
| Lima, 2015 (32) | Deterministic compartmental model of HIV transmission among black American MSM inside and outside of jail and prison | HIV-TTR and increasing condom use |
|
HIV incidence: 9,246 new cases and 2,585 deaths over 10 years |
|
|
||
| Altice, 2016 (13) | Deterministic compartmental model of HIV transmission among PWID inside and outside of prison | Prison-based OAT and maintaining treatment after release |
|
|
50% OAT coverage in prison with 12 months retention after release would reduce HIV prevalence in the community by 28% and cumulative incidence by 20% from 2015 to 2030. Without postrelease treatment retention, only 6% of new infections would be averted |
|
||
| Legrand, 2008 (35) | Stochastic compartmental model of TB transmission within prison | DOTS, systematic detection at entry point of symptomatic smear-positive cases, systematic detection at entry point using chest radiograph, annual mass screening of inmates by chest radiograph | TB prevalence 4.6% |
|
|
|||
| Cooper-Arnold, 1999 (53) | Deterministic model of TB transmission within urban jails | Decreasing time to diagnosis, and improving minimum ventilation to 12 ACH | TB infection risk: 13% risk of infection after 24 hours within the facilities | 64% increase in ventilation, in the lock-up, to reach design specifications (15 cfm) would reduce infection by 62.5% among sheriffs |
|
|||
| Winetsky, 2012 (34) | Deterministic model of TB transmission within prison | Screening and diagnosis strategy, alone or in combination: self-referral, symptom screening, MMR, and sputum PCR with probes for rifampin resistance (Xpert MTB/RIF; Cepheid Inc., Sunnyvale, CA) |
|
|
|
|||
| Urrego, 2015 (52) | Deterministic model of TB transmission within prison | Improved ventilation and early diagnosis through case finding | TB infection risk: 65%–81% risk of infection after exposure for 6 months to an infectious cellmate | Decreasing time to diagnosis by 25% resulted in a 8.3% reduction in transmission risk. Furthermore, improving ventilation to WHO cell occupancy standards reduced transmission by 38.2% and optimizing cross-ventilation decreased transmission by 64.4% |
|
|||
| Johnstone-Robertson, 2011 (51) | Deterministic model of TB transmission within prison | DOTS in combination with the implementation of national and international minimum standards for cell occupancy during incarceration | TB infection risk: 90% risk of infection after exposure of 60 days to an infectious cellmate | Implementing current national or international cell occupancy standards would reduce the risk of infection by 30% and 50%, respectively. Adding active case findings to the national and international standards of cell occupancy would reduce the risk of infection by 50% and 94%, respectively |
|
|||
| Scott, 2015 (58) | Deterministic compartmental model of HBV transmission inside prison | Condom distribution program, and opt-out STIs screening at prison entry | 1.26% HBV prevalence and an incidence of 7 new cases per year | Availability of condoms reduced annual incidence by 71% and prevalence by 6%. Combining condom availability with opt-out screening reduced incidence by 86% and prevalence by 50% |
|
|||
| Sutton, 2006 (19) | Deterministic compartmental model of HBV transmission in general population | HBV vaccination at reception into prison | 18% HBV prevalence among PWID with an annual incidence of 1,238 news cases per year | Increasing vaccination coverage at prison entry from 10% in 2003 to 33% in 2006 would have reduced incidence and prevalence among PWID by 65% and 50%, respectively, by 2014. Increasing coverage to 50% would have reduced incidence by 80% and prevalence by 60%. 66% coverage would have reduced incidence by 85% and prevalence by 68% |
|
|||
| Martin, 2013 (21) | Deterministic compartmental model of HCV transmission inside and outside of prison | Introduction of DBS testing in prison and in community addiction services | Chronic HCV infection prevalence of 35% among all PWID |
|
|
|||
| Martin, 2016 (22) | Deterministic compartmental model of HCV transmission inside and outside of prison | Scale-up of opt-out testing in prison:
|
|
|
Doubling HCV testing in prison (e.g., through opt-out testing) with short-course, IFN-free DAA treatment was cost-effective compared with status quo risk-based testing. The intervention is highly cost-effective if more than 10% of referred PWID are treated in prison |
|
||
| He, 2016 (20) | Stochastic individual-based model of HCV transmission inside and outside of prison | HCV screening and treatments in prisons. Screening scenarios included 1-time risk-based screening of currently incarcerated and entrants who were active or former IDUs for 1 year (1Yr-Risk), 1-time opt-out universal HCV screening of currently incarcerated inmates followed by opt-out screening of all incoming inmates for up to 1 year (1Yr-All), 5 years (5Yr-All), and 10 years (10Yr-All) | Chronic HCV infection prevalence of 17.6% among all prisoners | Chronic HCV prevalence of 1.57% in general population and 35% among PWID |
|
|
||
| Stone, 2017 (23) | Deterministic compartmental model of HCV transmission inside and outside of prison | HCV treatment for chronically infected PWID at prison entry:
|
|
|
|
|
||
| Gopalappa, 2013 (30) | Stochastic individual-based models of chlamydia and gonorrhea transmission inside and outside of jail |
|
7% prevalence for chlamydia and 4.6% prevalence for gonorrhea among male inmates | 0.41% prevalence for chlamydia and 0.06% for gonorrhea | Compared with symptom-based testing, screening all male arrestees and only those younger than 35 years would avert 556 and 491 infections among women, respectively, if screening is done during prison entry, and 1,100 and 995 cases, respectively, if screening is done early at prison entry |
|
||
| Owusu-Edusei, 2013 (59) | Deterministic compartmental model of chlamydia transmission inside and outside of jail | Jail-based screen-and-treat programs |
|
|
|
|
||
| Scott, 2015 (58) | Deterministic compartmental models of chlamydia, syphilis, and gonorrhea transmission within prison | Condom distribution program, and opt-out STI screening at prison entry | Prevalence:
|
|
|
|||
| Tuli, 2009 (29) | Deterministic compartmental models of chlamydia, gonorrhea, and syphilis transmission inside and outside of jail | Screening, treatment, and condom provision intervention for inmates of a segregated unit for MSM |
|
|
Intervention is cost-effective for reducing chlamydia and gonorrhea infections and is cost-saving for syphilis |
|
||
Abbreviations: ACH, air changes per hour; cfm, cubic feet per minute; DAA, direct-acting antiviral; DBS, dried blood spot; DOTS, directly observed treatment short-course; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HIV-TTR, HIV testing, treatment, and post-release retention in treatment; IDU, injecting drug user; IFN, Interferon; MDR-TB, multidrug-resistant tuberculosis; MMR, mass miniature radiography; MSM, men having sex with men; MTB/RIF, Mycobacterium tuberculosis/rifampicin; OAT, opioid agonist therapy; PCR, polymerase chain reaction; PE, prison entry; PWID, people who inject drugs; QALY, quality adjusted life year; STIs, sexually transmissible infections; SVR, sustained virologic response; TB, tuberculosis; WHO, World Health Organization.
Model assessments
Parameter estimation is fundamental to model development (15, 24). Model fitting to epidemiologic data was conducted in 14 of the 34 studies. Reported methods included least-squares approaches (21), approximate Bayesian computation (13, 23), Bayesian melding (4, 25), and maximum likelihood (19). Models most frequently were fitted to disease prevalence within incarcerated populations. Some models also were fitted to risk behaviors and incarceration rates data (21–23), and to data on disease incidence in prison (4, 26). Contrary to other fitting procedures that provide point estimates for model parameters, Bayesian inference approaches are used to derive probability distribution for model parameters from prior information on parameters and empirical data. Bayesian approaches provide a theoretically sound method to propagate the uncertainty of empirical data into the uncertainty estimates of model parameters and, subsequently, onto the uncertainty of model predictions. This propagation of empirical uncertainty increases the credibility and information value of a model’s predictions. In addition, model validation, in which model output is compared with data that were not used for parameters estimation, is fundamental to assessing the accuracy of a model’s projection to empirical data (24, 27). However, model validation was undertaken in only 1 (20) of the 34 studies.
Variation in parameters can affect model projections arising from real-world nonlinearity captured by mathematical dynamic transmission models. Sensitivity analysis should be performed to characterize the contribution of a model’s outputs to the variation of input parameters (24, 28). Researchers can use such an analysis to identify sources of parametric uncertainty, forecast ranges of plausible outcomes, and ascertain the robustness of the model predictions. Sensitivity analyses were undertaken in 26 of the 34 studies; researchers used methods such as scenario analysis (n = 6 studies), univariate analysis (n = 13), partial rank correlation coefficients (n = 8), analysis of covariance (n = 2), and probabilistic sensitivity analysis (n = 1).
Scenario analyses were used to evaluate the contribution of postincarceration risk behavior on disease transmission and the effectiveness of prison-based interventions within the general population (13, 19, 23). According to these analyses, elevated risk behavior among recently released PWID was a primary contributor of postincarceration-related HIV and HCV transmission in the general population (13, 23). Consequently, continuation of drug-treatment after release is instrumental to extending the effectiveness of prison-based interventions (13, 23). Scenario analysis also was used to investigate the effect of sexual activity in jails on the cost-effectiveness of screening, treatment, and condom provision among men having sex with men in US jails (29). The findings of this model indicated this intervention combination becomes more cost-effective as the level of sexual activity within jails increases and could even be cost-saving for syphilis and gonorrhea control (29), if prisoners are as sexual active in jail as they were in the general population.
Univariate sensitivity analysis was used to assess the influence of specific parameters, such as intervention coverage and infection risk, on the effectiveness of prison-based interventions (4, 20–22, 30–33). According to these analyses, limited access to HIV prevention measures in prisons may have exacerbated HIV transmission within correctional facilities (4, 32, 33). The provision of prevention measures such as prison-based OAT necessitates a high level of coverage and postrelease continuation to achieve a substantial reduction in HIV transmission among PWID (4). Increasing screening and treatment rates of HCV in prisons was shown to significantly improve the effectiveness and cost-effectiveness of prison-based interventions for reducing HCV transmission and burden in prison settings and in the general population in the United Kingdom and United States (20–22).
Probabilistic sensitivity analysis was used to identify the willingness-to-pay thresholds over which a given intervention strategy has the highest probability of being cost-effective compared with other strategies (34). Winetsky et al. (34) showed that above a willingness-to-pay threshold of US$2,500 per quality-adjusted life year, the use of sputum polymerase chain reaction for annual mass TB screening in prisons is cost-effective in Russia and Eastern European countries, where TB burden is elevated.
Partial rank correlation coefficients analysis and analysis of covariance were used to assess the effect of each parameter on a model’s predictions, while accounting for the combined contribution of all parameters to a given model outcome (4, 13, 31, 34, 35). These multivariate analyses broadly confirmed results of scenario analysis by consistently demonstrating that the elevated risk of infection among recently released PWID is the primary driver of the contribution of incarceration to HIV transmission in the general population (4, 13).
Human immunodeficiency virus
In the majority of articles reviewed, researchers had studied HIV transmitted by syringe sharing and unprotected sex between men. Prison environments may exacerbate risk of HIV transmission, given the high rates of incarceration among PWID and the lack of prevention measures, such as the provision of sterile injecting equipment and condoms (4, 36, 37). Moreover, incarceration has been associated with initiation of drug use or the transition from noninjection drug use to injection drug use (38).
In 14 of the 34 articles, authors reported on modeled HIV transmission dynamics (4, 13, 25, 29, 32, 33, 39–46). Authors of 11 of these 14 articles focused on HIV transmission within prisons (25, 29, 32, 39–46) and in 3 of these articles, researchers focused on HIV transmission among PWID in and out of prisons (4, 13, 33). In 9 articles, researchers reported on HIV transmission modeled within a single prison setting (25, 29, 33, 40, 43, 44, 46). The spread of HIV across multiple prisons (39, 41) was considered in 2 articles, and in 3, authors described transmission dynamics within and between prison and community settings (4, 13, 32). Authors of 4 articles evaluated the effectiveness of prison-based interventions such as screening with treatment (29, 32), condom distribution (29, 32), and OAT (4, 13).
A model of HIV transmission among incarcerated African-American men having sex with men and having a history of incarceration was used by Basu et al. (32) to evaluate the effectiveness of prison-based HIV testing, antiretroviral therapy treatment, and retention in long-term treatment HIV testing, treatment, and postrelease retention in treatment with or without provision of condoms in Atlanta, Georgia. In the model, the researchers accounted for the transition of individuals between the general community, jail, and prison, under the assumption that the behavior of men having sex with men was initiated within the community before incarceration (32). Setting-specific risks of infection were considered in this model, with a higher number of sexual partners and condom use in the community compared with jail and prison. Moreover, for this model, the researchers assumed the risk of infection within the general community was unaffected by an individual’s incarceration history (32). Based on the results of their model, Basu et al. (32) projected that expanding the HIV testing rate and antiretroviral therapy coverage to 80% while achieving 100% retention in long-term treatment would reduce cumulative HIV incidence and HIV-related death over a decade by 18% (from 77 to 63 cases) and 39% (from 18 to 12 deaths), respectively, within jails and by 8% (from 154 to 142 cases) and 18% (from 34 to 29 deaths), respectively, in prisons.
Two models were used to evaluate the effectiveness of prison-based OAT for controlling HIV transmission among PWID in prison and in the community (4, 13). The models accounted for HIV transmission through syringe sharing inside and outside of correctional facilities, but sexual transmission was not incorporated in the models (4, 13). Consistent with empirical data, the models were designed to use the assumption that incarceration is associated with an increase in the risk of infection among PWID with a history of incarceration compared with those who have never been incarcerated. For the first model, it was assumed that recently (released in the last 12 months) and previously (>12 months since release) incarcerated PWID had higher risks of infection than PWID who were never incarcerated (13). Recently incarcerated PWID were assumed to have a higher infection risk than previously incarcerated PWID (13). For the second model, it was assumed that only recently (released in the last 6 months) incarcerated PWID had a higher risk of infection (4).
The first model was focused on HIV transmission among PWID in Ukraine (13), taking into account that individuals could initiate injection drug use either in prison or within the community (13). According to model results, it was estimated that incarceration could contribute 55% (95% credible interval (CrI): 40, 68) of new HIV infections among PWID over 15 years (13). If only recently incarcerated individuals have a heightened risk of infection, incarceration would contribute 28% (95% CrI: 13, 41) of new infections (13). Achieving a 50% coverage of OAT in prison, with continuation of treatment during the first 12 months after release, would reduce community HIV incidence by 39% (95% CrI: 23, 49), from 2.9 to 1.8 cases per 100 person-years, and prevalence by 28% (95% CrI: 18, 36), from 20% to 14%, from 2015 to 2030 (13). Over 15 years, cumulative incidence in the population would decrease by 20% (95% CrI: 15, 25) (13). This reduction was primarily attributable to OAT retention upon release, because only 6% (95% CrI: 2, 8) of new infections were averted without retention in OAT (13).
For the other model, it was assumed that injection drug use was initiated within the general community and that a proportion of PWID who did not share syringes in the community were likely to initiate syringe sharing during incarceration (4). Prison-based OAT with continuation of treatment during the first 6 months after release was considered in this model, as well as postrelease discontinuation of antiretroviral therapy. The researchers assessed a diversity of scenarios for HIV prevalence among PWID (4) that considered HIV prevalence ranging from 5%, such as occurs in Turkey (47), to 60%, in Estonia (47), informed by empirical data on HIV prevalence among PWID (4). In these analyses, repercussions of incarceration on HIV incidence across the general community were assessed. In communities where HIV prevalence among PWID is greater than 20%, incarceration was estimated to contribute 12% (95% CrI: 0.5, 52) of new HIV infections outside of prison over 5 years. In communities where HIV prevalence among PWID is lower than or equal to 20%, it was projected that 21% (95% CrI: 0.4, 53) of new HIV infections in the general community are attributable exacerbated risks within prison (4). Based on the findings from the model, the authors suggested that achieving an 100% coverage of prison-based OAT, with postrelease maintenance of treatment, could avert 12% (95% CrI: 2, 45) of cumulative new HIV infections in communities with HIV prevalence greater than 20%, and 29% (95% CrI: 10, 58) in communities with HIV prevalence lower than or equal to 20%, over 5 years (4). Furthermore, reducing the incarceration rate of PWID by 50% would reduce the community-wide HIV incidence by 8% (5% CrI: 0, 27) in communities with HIV prevalence greater than 20%, and by 15% (95% CrI: 2, 30) in communities with HIV prevalence less than 20% (4). The contribution of incarceration to disease transmission and the benefit of reduced incarceration were lower in communities with high HIV prevalence, because of the underlying high risk of infection in the community.
Tuberculosis
Correctional facilities have been regarded as a potential reservoir for TB (48), with an average incidence in prison 23 times higher than that of the general population (49) and a prevalence reported to be as high as 100 times that of the general population (50). The spread of TB within prisons can be attributed to overcrowding, multiple prison transfers, poor ventilation, and low access to treatment. Understanding the dynamics of TB transmission in correctional facilities and its contribution to the spread of TB within local communities is important for informing public health efforts for TB control.
In our review, transmission of TB in prisons was modeled in 10 studies, with 2 models accounting for HIV-TB coinfection dynamics. Nine models were focused on the dynamics of TB transmission within prison, and a prison-community metapopulation model was used to consider the joint dynamics of TB infection within and outside prison.
Three models were used to evaluate the risk of TB transmission during incarceration and the effectiveness of changes in prison design for reducing TB incidence (51–53). The first model was used by Johnstone-Robertson et al. (51) to estimate the effectiveness of prison occupancy reforms on reducing TB transmission in South African prisons. The effectiveness of implementing the current national statutory minimum prison occupancy, or the World Health Organization recommendations for adequate ventilation, to reduce TB transmission in prison was evaluated. According to the model, under the national statutory minimum prison occupancy, transmission probability could be reduced by 30%, from 90% to 63% probability of infection, after exposure of 60 days or more to an infectious cellmate (51). The transmission probability could be reduced by 50% under World Health Organization recommendations for adequate ventilation (51). Adding active case finding to these interventions could reduce transmission by 50% (from 90% to 45% probability of infection) under the national statutory minimum prison occupancy, and 94% (from 90% to 5% probability of infection) under World Health Organization recommendations (51). The second model was used to evaluate the risk of TB transmission in 3 prisons in the state of Mato Grosso do Sul in Brazil (52). Using this model, Urrego et al. (52 showed that inmates have a 65% (95% confidence interval (CI): 55, 77) to 81% (95% CI: 75, 87) risk of infection after exposure of 180 days to an infectious cellmate. Based on the model, they estimated that improving ventilation to the World Health Organization recommended minimum ventilation rate of 60 L/s per person would decrease TB incidence in Brazilian prisons by 38%, and could be further reduced by 64% if cross-ventilation between prison cells was improved (52). The third model was used to evaluate the risk of infection for sheriffs during a TB outbreak within Connecticut urban jails (53). Using this model, researchers demonstrated that ensuring 15 cubic feet per minute for mean outside airflow per occupant could reduce TB transmission by 63% (from a 13% risk of infection after 24 hours within the facilities) (53).
Two models were used to evaluate the effectiveness of prison-based mass screening and treatment of TB (34, 35). According to the first model, a combination of annual radiographic mass screening, case detection upon prison entry, and directly observed treatment short-course could reduce TB prevalence in prison by 90% (from 4.6% to <0.5%) over 10 years in Rio de Janeiro (35). Using the second model, researchers showed that annual mass screening of all prisoners, using sputum polymerase chain reaction, followed by directly observed treatment short-course is a highly cost-effective strategy for reducing TB and multidrug-resistant tuberculosis (MDR-TB) prevalence within prisons in the former Soviet Union (34). The incremental cost-effectiveness ratio of this strategy was compared with that of mass miniature radiography screening, mass miniature radiography screening with sputum polymerase chain reaction for rapid MDR-TB detection, and combined mass miniature radiography and symptom screening with sputum polymerase chain reaction for rapid MDR-TB detection (34).
A metapopulation model was used to evaluate the effect reduction in the incarceration rate may have on TB incidence and the risk of propagating new drug-resistant TB strains in the general population (31). It was assumed that the risk of TB infection was higher in prison than in the general community and that the risk of infection within the general population was independent of the incarceration history of individuals. Using this model, researchers showed that decreasing the population at risk of incarceration from 3% to 2% of the general population would reduce TB and MDR-TB prevalence in prison by 44% (from 771 to 137 cases per 100,000 population) and 38% (from 13 to 8 cases per 100,000), respectively, and community prevalence by 21% (from 173 to 137 cases per 100,000) and 20% (from 5 to 4 cases per 100,000), respectively (31). The model also indicated that each 1% increase in the population at risk of incarceration would lead to a 4% increase of emergent MDR-TB strains (31).
Viral hepatitis
HCV and HBV are bloodborne diseases spread via contaminated needles related to drug use and tattooing within prisons. In contrast to HCV, sexual transmission is the primary route for HBV transmission. An estimated 4.8% of incarcerated individuals worldwide have chronic HBV and 15% are infected with HCV (4), whereas the general population prevalence of HBV is estimated to be 3.6% (54) and 2.8% for HCV (55). In developed countries, 90% of new HCV infections occur among PWID and between 56% and 90% of PWID have a history of incarceration, yet diagnosis and treatment among PWID and within correctional facilities remain low (21, 37). These factors make correctional facilities potential drivers for HBV and HCV epidemics within and outside prison. However, prisons provide an opportunity for curbing disease transmission through the provision of prison-based prevention, HBV vaccination, and HCV treatment. Mathematical models have been used to evaluate these potential roles of correctional facilities on both the transmission and control of HBV and HCV.
We retrieved 2 articles in which modeling of HBV was done and 7 articles in which HCV was modeled within correctional settings. In 3 of the reviewed articles, authors reported on modeled disease transmission dynamics within a single prison (42, 56, 57); in 2 studies, authors modeled a network of prisons (23, 58); and in 4 studies, the interactions between prison and the general community were modeled (19–22). Five of the models exclusively accounted for disease transmission through the sharing of syringes, 1 accounted for sexual transmission, and3 accounted for a combination of transmission routes. Of these 9 models, 8 (19–23, 44, 56, 57) were used to evaluate the effectiveness of prison-based intervention measures, including disease screening (n = 4), HCV treatment (n = 2), HBV vaccination (n = 1), condom distribution (n = 1), and health education (n = 2).
A model of HBV transmission among PWID was used to evaluate the effectiveness of HBV vaccination at prison entry in England and Wales (19). Using this model, researchers assumed that PWID had a constant infection risk irrespective of incarceration (19). The findings showed that scaling up vaccination from 10% in 2003 to 50% by 2006 would have reduced the incidence and prevalence of HBV among all PWID by 80% (from 1,238 to 247 new cases annually) and 60% (from 18% to 7%), respectively, by 2014 (19). If HBV vaccination at prison entry was scaled up to 66%, incidence and prevalence among all PWID were predicted to be reduced by 85% and 68%, respectively (19). In Australia, using a model of HBV transmission within a network of 14 prisons, researchers determined that condom distribution in prison would decrease HBV incidence in prisons by 71% (from 7 to 2 cases per year) (58).
A model of HCV transmission dynamics within prison and the general population was developed to evaluate the contribution of incarceration to HCV transmission in Scotland and the effectiveness of prison-based control measures (23). The model accounted for the association between incarceration and drug injecting behavior by assuming that recently released individuals had an increased risk of HCV infection during the first 6 months due to resumption in injecting (23). Moreover, a lower risk of infection in prison was assumed in this model, compared with the infection risk in the general community, and that the risk of HCV infection among PWID increases with the duration of injecting (23). According to model results, incarceration contributes 28% (95% CrI: 3, 51) of HCV transmission among PWID in Scotland (23). In addition, eliminating incarceration of PWID could reduce HCV incidence and chronic prevalence by 22% (95% CrI: 4.8, 38.5), from 15.6 to 12.2 cases per 100 person years, and 17% (95% CrI: 6.1, 27.9), from 37.6% to 31.2%, respectively, by 2030. Using this model, the authors showed that scaling-up prison-based HCV treatment to 80% of prison entrants would reduce incidence and prevalence by 55.8% (95% CrI: 49.3, 61.4), from 15.6 to 6.9 cases per 100 person years, and 55.9% (95% CrI: 51.1, 61.3), from 37.6% to 16.6%, respectively, by 2030 (23). Pairing HCV treatment with postrelease interventions, such as linking PWID to harm reduction services and housing support, could decrease incidence and chronic prevalence by 76% (95% CrI: 65.6, 82.2) and 74% (95% CrI: 61.8, 77.3), respectively, by 2030 (23).
In 3 articles, researchers evaluated the cost-effectiveness of prison-based HCV screening and treatment in the United States and United Kingdom using dynamic transmission models (20–22). An assumption underlying these models was that incarceration does not affect the risk behavior of PWID (20–22). In the first study, a mathematical model of HCV transmission between prison and the general population was developed to evaluate the cost-effectiveness of HCV dried blood spot screening in drug use disorder programs and prisons (21). Martin et al. (21) showed that dried blood spot screening would likely be cost-effective for increasing HCV case finding in community addiction services. In prisons, dried blood spot screening is cost-effective if there is at least a 40% continuation of treatment or care between community and prison for diagnosed patients in each of the settings (21). Using the same HCV model, authors of the second article showed that introducing an opt-out approach to HCV testing in UK prisons, with an 8- to 12-week, interferon-free, direct-acting antivirals treatment, would be cost-effective compared with the status quo of voluntary risk-based testing paired with the recommended 8–24 weeks of therapy (22). These interventions were cost-effective if more than 10% of referred PWID were treated in prison compared with the current 2.5% (22). In the third study, the authors used an agent-based model of HCV transmission in the United States to demonstrate that the implementation of risk-based and opt-out screening in prisons followed by treatment with oral direct-acting antivirals would be cost-effective for reducing the health burden of HCV in that country (20).
Sexually transmissible infections
We identified 5 articles on dynamic transmission modeling of other sexually transmissible infections in correctional settings, with 4 models of chlamydia (29, 30, 58, 59), 3 of syphilis (29, 58, 59), and 3 of gonorrhea (29, 30, 58). Three of the articles were focused on disease transmission within a single prison (29, 30, 58), 1 on multiple prisons (58), and 1 on transmission between prison and the general community (59). Using the model of chlamydia transmission between prison and the general community, researchers focused on heterosexual transmission with the assumption of no disease transmission within prison (59). The effectiveness of prison-based intervention strategies, such as sexually transmissible infections screening at prison entry (58), condom distribution (29, 58), and treatment programs (29, 59), were evaluated in 4 of the articles.
Mathematical modeling analyses showed that jail-based screening and treatment programs in the United States could reduce the prevalence of chlamydia by 13% (from 2.3% to 2.0%) in communities with low incarceration rates (0.6%) and by 54% (from 4.6% to 2.1%) in communities with higher incarceration rates (11%) (59). When paired with condom provision, jail-based screening and treatment were cost-effective for averting chlamydia and gonorrhea infections and cost-saving for averting syphilis among men having sex with men in Los Angeles, California, (29). Combining sexually transmissible infections screening at prison entry with condom provision was shown to reduce chlamydia, syphilis, and gonorrhea incidence by 31% (from 715 to 493 new infections per year), 98% (from 67 to 1 new infection per year), and 99% (from 115 to 1 new infection per year), respectively, in Australia (58).
Methicillin-resistant S. aureus
MRSA is a staphylococcal bacterium that is transmitted primarily through contact with an infected wound, the sharing of items that have come in contact with infected skin, or any other circumstances that involve skin-to-skin contact. In 2 of the reviewed studies, researchers modeled MRSA transmission within correctional facilities (26, 60). A dynamic transmission model was used to investigate the effect of community-acquired MRSA on the spread within a correctional facility in Los Angeles and to determine if reincarceration significantly affects transmission within prison disease dynamics (60). Based on the model results, the study authors reported that decreasing rates of reincarceration would reduce the prevalence of secondary infections acquired within prison. Specifically, decreasing the probability of reincarceration by 50% was predicted to reduce the prevalence of MRSA within prison by 25% (60).
DISCUSSION
Mathematical models have been used to evaluate the spread and control of infectious diseases within and between correctional facilities and the general population. Modeling analyses have quantified the role of incarceration in disease transmission with the general community, as well as the effectiveness and cost-effectiveness of prison-based interventions. Specifically, model results were used to estimate that in communities where HIV prevalence among PWID is greater than 5%, incarceration contributes 12%–55% of infections (4, 13). This contribution is driven by high incarceration rates, postrelease increased-risk behavior, and discontinuation of treatment among PWID (4, 13, 23). Furthermore, prison-based OAT, combined with postrelease continuation of treatment, was predicted to be a highly effective strategy for reducing HIV incidence and prevalence among PWID (4, 13). Using the models, study authors showed that reducing incarceration rates among PWID would decrease HIV transmission within prisons and the general population (4, 13). For HCV, the provision of prison-based opt-out universal testing and treatment was assessed to be cost-effective in the United Kingdom and the United States (20–22). Similarly, it was shown that prison-based screening followed by directly observed treatment short-course for treatment is cost-effective for reducing TB and MDR-TB prevalence among prisoners in the former Soviet Union (34). Decreasing the proportion of the population at risk of incarceration by 33% could reduce TB and MDR-TB by at least 21% and 20%, respectively, in a TB-endemic community (31). Treating nonviolent drug offenders and substance abuse disorders as medical rather than criminal issues would reduce incarceration, and would more effectively and cost-effectively address substance use disorders (12).
Our review of model parameterization, calibration, validation, and sensitivity and uncertainty analyses revealed that only 14 of the 34 articles were fitted to any epidemiologic data. Of the 34 articles, the impact of variation of input parameters on models’ outcomes was evaluated in 26, and in 14 articles, researchers accounted for the effect of parameter value uncertainty on the outcomes of models (Table 3). Only 8 of the 34 studies included model fitting, uncertainty, and sensitivity analysis, with only 1 including model validation (Table 3). These results highlight the need for more modeling studies to include model calibration and validation against empirical data, as well as uncertainty and sensitivity analyses. These analyses are essential for assessing confidence in the appropriateness of the model structure and outcomes (15, 24).
Table 3.
Summary of All Included Articles (n = 34), 1996–2017
| First Author, Year (Reference No.) | Disease | Modeled Population | Modeled Setting | Model Fitting | Model Validation | Sensitivity and Uncertainty Analyses |
|---|---|---|---|---|---|---|
| Ching, 2007 (39) | HIV | All inmates | Network of prisons | None | None | Scenario analysis |
| Dolan, 1998 (33) | HIV | PWID inmates | Single prison | None | None | Univariate sensitivity analyses |
| Gani, 1999 (40) | HIV | HIV-positive inmates | Single prison | None | None | None |
| Yakowitz, 1996 (41) | HIV | All inmates | Multiple prisons | None | None | Univariate sensitivity analysis |
| Dolan, 2016 (4) | HIV | PWID and non-PWID | Prison-community metapopulation | Bayesian melding | None | Univariate sensitivity analysis and PRCC analysis |
| Pinkerton, 2007 (43) | HIV | Male inmates | Single prison | None | None | Scenario analysis and univariate sensitivity analysis |
| Lima, 2015 (32) | HIV | Black MSM, 15–54 years old | Prison-community metapopulation | 2-sample Kolmogorov-Smirnov test | None | Univariate sensitivity analysis |
| Altice, 2016 (13) | HIV | PWID and non-PWID | Prison-community metapopulation | Approximate Bayesian computation sequential Monte Carlo scheme | None | Analysis of covariance |
| Gani, 1997 (46) | HIV | All inmates | Single prison with extension to 2-prison system | None | None | None |
| Mushayabasa, 2011 (44) | HIV | Male inmates | Single prison | None | None | Univariate sensitivity analysis: normalized forward sensitivity index |
| Basu, 2011 (31) | TB | Inmates and general population | Prison-community metapopulation | None | None | Univariate sensitivity analysis and PRCC |
| Cooper-Arnold, 1999 (53) | TB | Deputy sheriffs | Single prison | None | None | Univariate sensitivity analysis |
| Legrand, 2008 (35) | TB | All inmates | Single prison | Manual calibration | None | Latin hypercube sampling and PRCC analysis |
| Mushayabasa, 2011 (66) | TB | All inmates | Single prison | None | None | Latin hypercube sampling and PRCC analysis |
| Herrera, 2013 (65) | TB | All inmates | Single, semiclosed setting (prison as an example) | None | None | None |
| Winetsky, 2012 (34) | TB | All inmates | Single prisons, without interaction between them | Manual calibration | None | Multiple: 1) univariate sensitivity analyses, 2) selected 2-way sensitivity analyses, 3) probabilistic sensitivity analysis, and 4) scenario analyses |
| Urrego, 2015 (52) | TB | All inmates | Per prison cell, data from 3 prisons used | None | None | Scenario analysis |
| Johnstone-Robertson, 2011 (51) | TB | All inmates | Single prison | None | None | None |
| Mushayabasa, 2011 (57) | HCV | Female PWID inmates | Single prison | None | None | PRCC analysis |
| Martin, 2013 (21) | HCV | PWID and non-PWID | Prison-community metapopulation | Least-squares approach | None | 1-way sensitivity analysis and probabilistic uncertainty analysis |
| Martin, 2016 (22) | HCV | PWID and non-PWID | Prison-community metapopulation | Least-squares approach | None | Univariate sensitivity analyses and probabilistic uncertainty analysis |
| Mushayabasa, 2013 (56) | HCV | All inmates, with subcategorization into PWID and non-PWID | Single prison | None | None | Latin hypercube sampling and PRCC analysis |
| He, 2016 (20) | HCV | PWID and non-PWID | Prison-community metapopulation | Goodness-of-fit metric: relative distance within 5% of target value |
|
Univariate sensitivity analysis |
| Stone, 2017 (23) | HCV | PWID | Prison-community metapopulation | Approximate Bayesian computation | None | Analysis of covariance |
| Sutton, 2006 (19) | HBV | PWID and non-PWID | Prison-community metapopulation | Maximum likelihood | None | Scenario analysis |
| Gopalappa, 2013 (30) | STIs (chlamydia and gonorrhea) | Male inmates | Single prison | None | None | Univariate sensitivity analysis |
| Owusu-Edusei, 2013 (59) | STI (chlamydia) | Inmates, aged 18–35 years | Prison-community metapopulation | None | None | Latin hypercube sampling and PRCC analysis |
| Beauparlant, 2016 (60) | MRSA | Inmates and general population | Prison-community metapopulation | None | None | Latin hypercube sampling and PRCC analysis |
| Kajita, 2007 (26) | MRSA | Male inmates | Single facility (jail) | No details provided on methodology | None | None |
| Tuli, 2009 (29) | HIV and STIs (syphilis, chlamydia, gonorrhea) | MSM in segregated unit | Segregated unit in single prison | None | None | Scenario analysis |
| Hotta, 2010 (25) | HIV and TB | All female inmates | Single prison | Bayesian melding | None | None |
| Burattini, 2000 (42) | HIV, HCV, STI (syphilis) | All inmates | Single prison | Least-squares approach | N/A | None |
| Scott, 2015 (58) | HIV, HBV, and STIs (syphilis, chlamydia, and gonorrhea) | Male inmates | Network of 14 Victorian prisons | None | None | Monte Carlo uncertainty analysis and credible intervals obtained for outcomes |
| Raimundo, 2002 (45) | HIV, TB with possible coinfection | Female inmates | Single prison | Nonlinear least-squares estimation method | None | Univariate sensitivity analysis |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MSM, men having sex with men; PRCC, partial rank correlation coefficient; PWID, people who inject drugs; STI, sexually transmissible infection; TB, tuberculosis.
Mathematical modeling is an essential tool for answering key public health questions that traditional epidemiologic studies may not be able to address because of operational or ethical constraints (61). For example, molecular epidemiologic approaches could theoretically be used to evaluate the contribution of postrelease risk of infection to disease incidence among hidden populations, such as PWID, but would be very challenging operationally. As an alternative, mathematical modeling has been used to provide timely assessments of the effectiveness and cost-effectiveness of prison-based and targeted postrelease interventions for disease control. In addition, mathematical models can also be used to evaluate the potential effect of criminal justice reforms (such as diversion of nonviolent, substance-abusing offenders to community-based treatment) on disease transmission within prisons and the general community.
Empirical data used for model parameterization, calibration, and validation underlie the robustness of model projections. Most of the reviewed models were only fitted to single data point estimates of disease prevalence in correctional facilities, with the exception of 2 models that were also fitted to incidence data (Table 3). Exclusive model fitting to prevalence point estimates conducted in most of the modeling studies reviewed was likely due to the sparsity of data on disease incidence within prisons (4). Model fitting to prison’s prevalence point estimates was more often complemented by prevalence and incidence data from the general population, which are available more often. However, these models may yield erroneous estimates of the risk of infection within correctional facilities. Similarly, data on the extent to which current or prior incarceration increases risks of infection for individuals are limited. These empirical uncertainties could be informed by collection of longitudinal data on disease prevalence at prison entry and release, data on risk behavior (e.g., sexual and drug-use behaviors) within correctional facilities, and on postrelease risk behavior and treatment adherence. Such data collection would have its own challenges and limitations. Although prevalence data collected on prison entry and release, for example, would inform disease incidence in prison, prisoners with shorter sentences would be overrepresented (62). Thus, if duration of sentence is not taken into account in the statistical analyses, bias would arise. In addition, data collection at the individual level may present some operational challenges given that prisoners are often transferred between prisons before release or re-enter different communities from the ones they left when incarcerated.
Our systematic review focused on peer-reviewed articles written in English. Consequently, additional models published in other languages may have been missed. We also focused exclusively on dynamic transmission models, excluding other mathematical models, such as Markov models, which do not account for the mechanism of disease transmission. In the course of our review, we did not identify any modeling studies that considered hepatitis A virus, influenza, measles, and gastroenteritis, despite the burden they pose within correctional facilities (63).
There has been limited use of modeling disease transmission within correctional facilities compared with other institutional settings such as hospitals (64), although prisons are serving as reservoirs for infectious diseases in the general population (4). With the growing incarceration epidemic in many countries, high burden of diseases among prisoners, and interdependence with disease transmission in the general populations, there is an urgent need for evidence-based public policy on optimal intervention measures for disease control in correctional settings and the general population.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Center for Infectious Disease Modeling and Analysis, Yale School of Public Health, Yale University, New Haven, Connecticut (Martial L. Ndeffo-Mbah, Vivian S. Vigliotti, Laura A. Skrip, Alison P. Galvani); Department of Epidemiology and Microbial Disease, Yale School of Public Health, Yale University, New Haven, Connecticut (Martial L. Ndeffo-Mbah, Vivian S. Vigliotti, Laura A. Skrip, Alison P. Galvani); and Program of International Research and Training, National Drug and Alcohol Research Centre, University of New South Wales, Sydney, New South Wales, Australia (Kate Dolan).
The study was funded by the National Institutes of Health grants U01 GM087719 and U01 GM105627.
Conflict of interest: none declared.
Abbreviations
- CrI
credible interval
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- MDR-TB
multidrug-resistant tuberculosis
- MRSA
methicillin-resistant Staphylococcus aureus
- OAT
opioid agonist therapy
- PWID
people who inject drugs
- TB
tuberculosis
REFERENCES
- 1. Walmsley R. World Prison Population List. 2016. http://prisonstudies.org/sites/default/files/resources/downloads/world_prison_population_list_11th_edition_0.pdf. Accessed April 4, 2017.
- 2. Wildeman C, Wang EA. Mass incarceration, public health, and widening inequality in the USA. Lancet. 2017;389(10077):1464–1474. [DOI] [PubMed] [Google Scholar]
- 3. Pettit B, Western B. Mass imprisonment and the life course: race and class inequality in US incarceration. Am Sociol Rev. 2004;69(2):151–169. [Google Scholar]
- 4. Dolan K, Wirtz AL, Moazen B, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet. 2016;388(10049):1089–1102. [DOI] [PubMed] [Google Scholar]
- 5. Niveau G. Prevention of infectious disease transmission in correctional settings: a review. Public Health. 2006;120(1):33–41. [DOI] [PubMed] [Google Scholar]
- 6. Bick JA. Infection control in jails and prisons. Clin Infect Dis. 2007;45(8):1047–1055. [DOI] [PubMed] [Google Scholar]
- 7. Hammett TM, Harmon MP, Rhodes W. The burden of infectious disease among inmates of and releasees from US correctional facilities, 1997. Am J Public Health. 2002;92(11):1789–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rich JD, Beckwith CG, Macmadu A, et al. Clinical care of incarcerated people with HIV, viral hepatitis, or tuberculosis. Lancet. 2016;388(10049):1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamarulzaman A, Reid SE, Schwitters A, et al. Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. Lancet. 2016;388(10049):1115–1126. [DOI] [PubMed] [Google Scholar]
- 10. Deiss RG, Rodwell TC, Garfein RS. Tuberculosis and illicit drug use: review and update. Clin Infect Dis. 2009;48(1):72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Degenhardt L, Whiteford HA, Ferrari AJ, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1564–1574. [DOI] [PubMed] [Google Scholar]
- 12. Macarayan E, Ndeffo-Mbah M, Beyrer C, et al. Philippine drug war and impending public health crisis. Lancet. 2016;388(10062):2870. [DOI] [PubMed] [Google Scholar]
- 13. Altice FL, Azbel L, Stone J, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016;388(10050):1228–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lessler J, Cummings DA. Mechanistic models of infectious disease and their impact on public health. Am J Epidemiol. 2016;183(5):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garnett GP, Cousens S, Hallett TB, et al. Mathematical models in the evaluation of health programmes. Lancet. 2011;378(9790):515–525. [DOI] [PubMed] [Google Scholar]
- 16. Jit M, Brisson M. Modelling the epidemiology of infectious diseases for decision analysis: a primer. Pharmacoeconomics. 2011;29(5):371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lugnér AK, Mylius SD, Wallinga J. Dynamic versus static models in cost-effectiveness analyses of anti-viral drug therapy to mitigate an influenza pandemic. Health Econ. 2010;19(5):518–531. [DOI] [PubMed] [Google Scholar]
- 18. Ibuka Y, Paltiel AD, Galvani AP. Impact of program scale and indirect effects on the cost-effectiveness of vaccination programs. Med Decis Making. 2012;32(3):442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sutton AJ, Gay NJ, Edmunds WJ. Modelling the impact of prison vaccination on hepatitis B transmission within the injecting drug user population of England and Wales. Vaccine. 2006;24(13):2377–2386. [DOI] [PubMed] [Google Scholar]
- 20. He T, Li K, Roberts MS, et al. Prevention of hepatitis C by screening and treatment in US prisons. Ann Intern Med. 2016;164(2):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin NK, Hickman M, Miners A, et al. Cost-effectiveness of HCV case-finding for people who inject drugs via dried blood spot testing in specialist addiction services and prisons. BMJ Open. 2013;3(8):e003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin NK, Vickerman P, Brew IF, et al. Is increased hepatitis C virus case-finding combined with current or 8-week to 12-week direct-acting antiviral therapy cost-effective in UK prisons? A prevention benefit analysis. Hepatology. 2016;63(6):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stone J, Martin NK, Hickman M, et al. Modelling the impact of incarceration and prison-based hepatitis C virus (HCV) treatment on HCV transmission among people who inject drugs in Scotland. Addiction. 2017;112(7):1302–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pitman R, Fisman D, Zaric GS, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–5. Value Health. 2012;15(6):828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hotta LK. Bayesian melding estimation of a stochastic SEIR model. Math Popul Stud. 2010;17(2):101–111. [Google Scholar]
- 26. Kajita E, Okano JT, Bodine EN, et al. Modelling an outbreak of an emerging pathogen. Nat Rev Microbiol. 2007;5(9):700–709. [DOI] [PubMed] [Google Scholar]
- 27. Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Value Health. 2012;15(6):843–850. [DOI] [PubMed] [Google Scholar]
- 28. Ndeffo Mbah ML, Medlock J, Meyers LA, et al. Optimal targeting of seasonal influenza vaccination toward younger ages is robust to parameter uncertainty. Vaccine. 2013;31(30):3079–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuli K, Kerndt PR. Preventing sexually transmitted infections among incarcerated men who have sex with men: a cost-effectiveness analysis. Sex Transm Dis. 2009;36(2 suppl):S41–S48. [DOI] [PubMed] [Google Scholar]
- 30. Gopalappa C, Huang YL, Gift TL, et al. Cost-effectiveness of screening men in Maricopa County jails for chlamydia and gonorrhea to avert infections in women. Sex Transm Dis. 2013;40(10):776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Basu S, Stuckler D, McKee M. Addressing institutional amplifiers in the dynamics and control of tuberculosis epidemics. Am J Trop Med Hyg. 2011;84(1):30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lima VD, Graf I, Beckwith CG, et al. Correction: the impact of implementing a test, treat and retain HIV prevention strategy in Atlanta among black men who have sex with men with a history of incarceration: a mathematical model. PLoS One. 2015;10(5):e0128734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dolan K, Wodak A, Hall W, et al. A mathematical model of HIV transmission in NSW prisons. Drug Alcohol Depend. 1998;50(3):197–202. [DOI] [PubMed] [Google Scholar]
- 34. Winetsky DE, Negoescu DM, DeMarchis EH, et al. Screening and rapid molecular diagnosis of tuberculosis in prisons in Russia and Eastern Europe: a cost-effectiveness analysis. PLoS Med. 2012;9(11):e1001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Legrand J, Sanchez A, Le Pont F, et al. Modeling the impact of tuberculosis control strategies in highly endemic overcrowded prisons. PLoS One. 2008;3(5):e2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hammett TM, Gaiter JL, Crawford C. Reaching seriously at-risk populations: health interventions in criminal justice settings. Health Educ Behav. 1998;25(1):99–120. [DOI] [PubMed] [Google Scholar]
- 37. Jürgens R, Ball A, Verster A. Interventions to reduce HIV transmission related to injecting drug use in prison. Lancet Infect Dis. 2009;9(1):57–66. [DOI] [PubMed] [Google Scholar]
- 38. Fazel S, Bains P, Doll H. Substance abuse and dependence in prisoners: a systematic review. Addiction. 2006;101(2):181–191. [DOI] [PubMed] [Google Scholar]
- 39. Ching WK, Cong Y, Ng TW, et al. A fast algorithm for the spread of HIV in a system of prisons. Math Comput Model. 2007;46(9–10):1247–1255. [Google Scholar]
- 40. Gani J. A note on the control of HIV in prisons. Environmetrics. 1999;10(6):677–683. [Google Scholar]
- 41. Yakowitz S, Blount M, Gani J. Computing marginal expectations for large compartmentalized models with application to AIDS evolution in a prison system. IMA J Math Appl Med Biol. 1996;13(4):223–244. [PubMed] [Google Scholar]
- 42. Burattini M, Massad E, Rozman M, et al. Correlation between HIV and HCV in Brazilian prisoners: evidence for parenteral transmission inside prison. Rev Saude Publica. 2000;34(5):431–436. [DOI] [PubMed] [Google Scholar]
- 43. Pinkerton SD, Galletly CL, Seal DW. Model-based estimates of HIV acquisition due to prison rape. Prison J. 2007;87(3):295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mushayabasa S, Bhunu CP. Modeling HIV transmission dynamics among male prisoners in sub-saharan Africa. Int J Appl Math. 2011;41:62–67. [Google Scholar]
- 45. Raimundo SM, Yang HM, Bassanezi RC, et al. The attracting basins and the assessment of the transmission coefficients for HIV and M. tuberculosis infections among women inmates. J Biol Syst. 2002;10(1):61–83. [Google Scholar]
- 46. Gani J, Blount M, Yakowitz S. The spread and quarantine of HIV infection in a prison system. SIAM J Appl Math. 1997;57(6):1510–1530. [Google Scholar]
- 47. Jolley E, Rhodes T, Platt L, et al. HIV among people who inject drugs in Central and Eastern Europe and Central Asia: a systematic review with implications for policy. BMJ Open. 2012;2(5):e001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sacchi FP, Praça RM, Tatara MB, et al. Prisons as reservoir for community transmission of tuberculosis, Brazil. Emerg Infect Dis. 2015;21(3):452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baussano I, Williams BG, Nunn P, et al. Tuberculosis incidence in prisons: a systematic review. PLoS Med. 2010;7(12):e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. World Health Organization Tuberculosis in prisons. 2016. http://www.who.int/tb/areas-of-work/population-groups/prisons-facts/en/. Accessed March 9, 2017.
- 51. Johnstone-Robertson S, Lawn SD, Welte A, et al. Tuberculosis in a South African prison – a transmission modelling analysis. S Afr Med J. 2011;101(11):809–813. [PMC free article] [PubMed] [Google Scholar]
- 52. Urrego J, Ko AI, da Silva Santos Carbone A, et al. The impact of ventilation and early diagnosis on tuberculosis transmission in Brazilian prisons. Am J Trop Med Hyg. 2015;93(4):739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cooper-Arnold K, Morse T, Hodgson M, et al. Occupational tuberculosis among deputy sheriffs in Connecticut: a risk model of transmission. Appl Occup Environ Hyg. 1999;14(11):768–776. [DOI] [PubMed] [Google Scholar]
- 54. Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. [DOI] [PubMed] [Google Scholar]
- 55. Thrift AP, El-Serag HB, Kanwal F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat Rev Gastroenterol Hepatol. 2017;14(2):122–132. [DOI] [PubMed] [Google Scholar]
- 56. Mushayabasa S, Bhunu CP, Magombedze G, et al. On the role of screening and educational campaigns on controlling HCV in correctional institutions. J Biol Syst. 2013;21(1):1350007. [Google Scholar]
- 57. Mushayabasa S, Bhunu CP, Smith RJ. Assessing the impact of educational campaigns on controlling HCV among women in prison settings. Commun Nonlinear Sci Numer Simul. 2011;17(4):1714–1724. [Google Scholar]
- 58. Scott N, McBryde E, Kirwan A, et al. Modelling the impact of condom distribution on the incidence and prevalence of sexually transmitted infections in an adult male prison system. PLoS One. 2015;10(12):e0144869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Owusu-Edusei K Jr, Gift TL, Chesson HW, et al. Investigating the potential public health benefit of jail-based screening and treatment programs for chlamydia. Am J Epidemiol. 2013;177(5):463–473. [DOI] [PubMed] [Google Scholar]
- 60. Beauparlant M, Smith R. A metapopulation model for the spread of MRSA in correctional facilities. Infect Dis Model. 2016;1(1):11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gingras G, Guertin MH, Laprise JF, et al. Mathematical modeling of the transmission dynamics of Clostridium difficile infection and colonization in healthcare settings: a systematic review. PLoS One. 2016;11(9):e0163880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Teutsch S, Luciani F, Scheuer N, et al. Incidence of primary hepatitis C infection and risk factors for transmission in an Australian prisoner cohort. BMC Public Health. 2010;10:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. World Health Organization Prisons and Health 2014. 2014. http://www.euro.who.int/__data/assets/pdf_file/0005/249188/Prisons-and-Health.pdf. Accessed April 3, 2017.
- 64. van Kleef E, Robotham JV, Jit M, et al. Modelling the transmission of healthcare associated infections: a systematic review. BMC Infect Dis. 2013;13:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Herrera M, Bosch P, Nájera M, et al. Modeling the spread of tuberculosis in semiclosed communities. Comput Math Methods Med. 2013;2013:648291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mushayabasa S, Bhunu CP, Simmonds F, et al. Transmission in prison settings. Far East J Appl Math. 2011;57:49–60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.