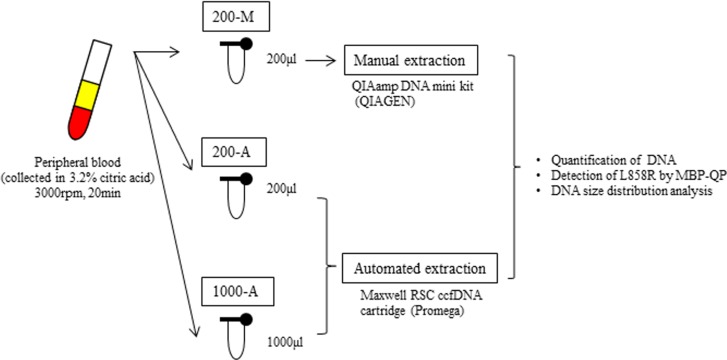
Figure 1. The design of this study.
We collected peripheral blood into tubes containing 3.2% citric acid. Blood was obtained from patients with advanced NSCLC (N = 61) and healthy volunteers (N = 10). Among 61 patients, 41 were verified to harbor EGFR L858R from tissue, and 20 were verified to not carry. After collection, blood samples were immediately centrifuged and the plasma from each tube was separated into two 200 μl aliquots and one 1000 μl aliquot. One 200 μl aliquot was subjected to manual plasma DNA extraction (200-M), the other 200 μl aliquot to automated extraction (200-A), and the 1000 μl aliquot to automated extraction (1000-A). The plasma volume for manual extraction was fixed by the limit of capacity with the QIAamp DNA mini Kit (QIAGEN).