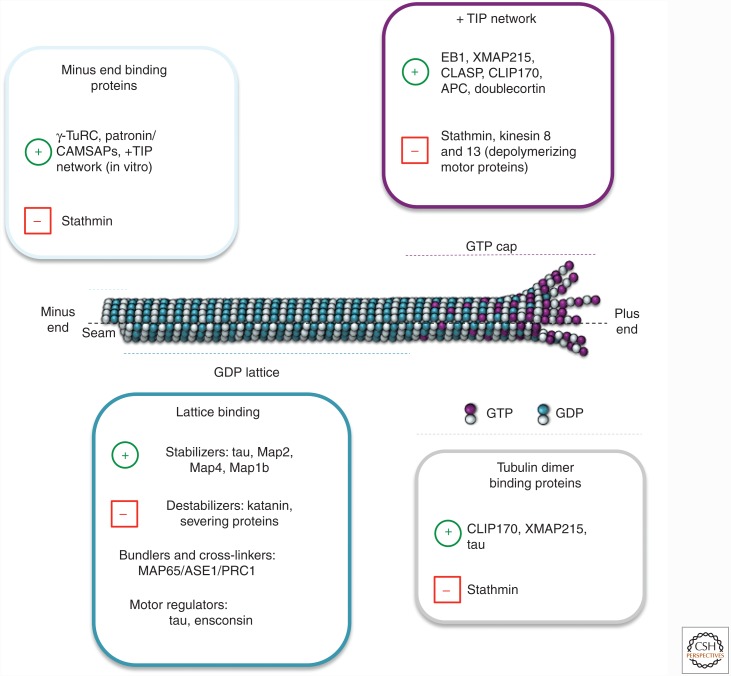
Figure 4.
Microtubule-binding proteins. Model summarizing some of the major microtubule-binding proteins according to their localization on the microtubule and their activities. The green plus symbol (+) means positive regulation and the red minus symbol (–) means negative regulation. At the plus end (fast growing), the members of the +TIP network (EB1, XMAP215, CLASP, CLIP170, doublecortin, and others not shown) associate with the stabilizing (GTP or GDP-Pi) cap of the growing microtubule and stabilize this dynamic structure to promote growth. Conversely, proteins such as the depolymerizing kinesins and stathmin facilitate microtubule disassembly. At the minus end (slow growing), proteins such as γ-TURC and Patronin/CAMSAP associate with the α-tubulin subunit to cap the end of the filament to prevent depolymerization, which is promoted by stathmin. In the central part of the microtubule, the GDP microtubule lattice can be stabilized by the activities of classical MAPs (tau, Map2, Map4, stop proteins) or destabilized by severing proteins (e.g., katanin). Microtubule-binding proteins that regulate the activity of microtubule motors also bind along the GDP lattice. Microtubules can form large networks through the activities of bundlers/cross-linkers, such as MAP65/ASE1/PRC1. Tubulin dimer binding proteins include stathmin (which promotes depolymerization by sequestering tubulin), as well as CLIP-170, tau, and XMAP-215 (which promote polymerization). Further detail and discussion are available in the main text and references.