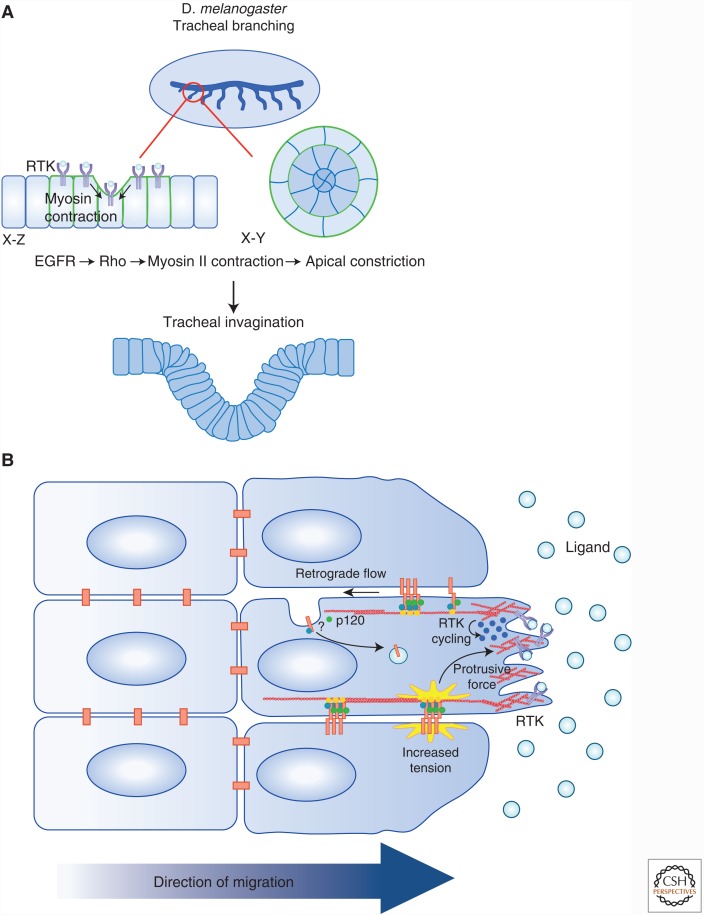
Figure 2.
RTK regulation of cell–cell junctions has tissue-scale impacts. Increasing evidence suggests that RTKs regulate cell communication during morphogenesis and collective cell migration. (A) During the development of the tracheal system in Drosophila, epithelial invagination is driven by the coordination between cytoskeletal rearrangements and mitosis. A wave of EGFR activity is required for the localization of MyosinII (green) at cell–cell boundaries to form multicellular arcs. Actomyosin contraction along these arcs (arrows) leads to apical constriction and the intercalation of cells into concentric circles, and they invaginate. (B) Collective cell migration is dependent on the dynamic modulation of adherens junctions and is tightly coordinated with RTK activity. RTK signaling in the leading cell stabilizes protrusive forces that exert tension on AJs in a polarized fashion, thereby reinforcing the forward protrusion of the cell. Additionally, evidence suggests that collectively migrating astrocytes show a gradient of cadherin endocytosis that leads to the treadmilling of adherens junctions along the lateral boundaries of migrating cells. This gradient is mediated by glycogen synthase kinase (GSK)-induced p120 phosphorylation, which promotes cadherin internalization and polarized trafficking to the leading edge.