Table 1.
Marine sources and compounds with potential for anti-prostate cancer drug development.
| Source Group | Compounds | Structure | Biological Activity on Prostate Cancer | References |
|---|---|---|---|---|
| Bacteria | Kahalalide F | 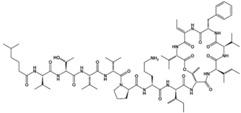 |
Cytotoxicity (IC50: 0.07 μM in PC-3 cells; 0.28 μM in DU-145 cells) 50% of PSA decline for ≥4 weeks at 80 μg/kg/day in clinical trial |
[14] [15] |
| Marine fungi | Demethoxyfumitremorgin C | 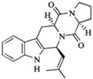 |
Inhibition of proliferation (50% inhibition at 100 μM in PC-3 cells) | [16] |
| Apochalasin V | 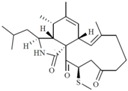 |
Cytotoxicity (IC50: 30.4 μM in PC-3 cells) | [17] | |
| Marine sponges | Rhizochalin | 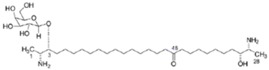 |
Cytotoxicity (IC50: 16.55 μM in PC-3 cells, IC50: 10.75 μM in DU-145 cells, IC50: 7.88 μM in LNCaP cells, IC50: 7.37 μM in 22Rv1 cells, IC50: 5.81 μM in VCaP cells) | [18] |
| Rhizochalinin | 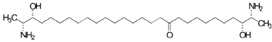 |
Cytotoxicity (IC50: 1.14 μM in PC-3 cells, IC50: 1.05 μM in DU-145 cells, IC50: 1.69 μM in LNCaP cells, IC50: 0.87 μM in 22Rv1 cells, IC50: 0.42 μM in VCaP cells) | [19] | |
| latrunculin A | 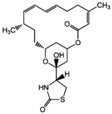 |
Inhibition of invasion (23% inhibition at 100 nM in PC-3 cells) | [20] | |
| Halichondramide | 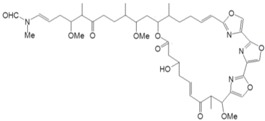 |
Cytotoxicity (IC50: 0.81 μM in PC-3 cells) | [21] | |
| Spongistatin 1 | 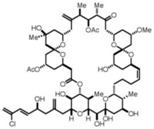 |
Inhibition of proliferation (50% inhibition at 500 pmol in LNCaP cells) | [22] | |
| Furospinosulin-1 | 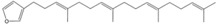 |
Inhibition of proliferation (60% inhibition at 100 μM in DU-145 cells) | [23] | |
| Sodwanone and Yardenone | 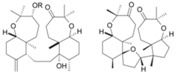 |
Inhibition of HIF-1α expression at 15 μM in PC-3 cells | [24] | |
| Niphatenone B | 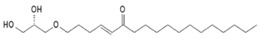 |
Inhibition of proliferation (90% inhibition at 250 μM in LNCaP cells) | [25] | |
| Agelasine B | 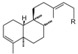 |
Cytotoxicity (IC50: 0.04 μg/mL in DU-145 cells) | [26] | |
| Cyanobacteria | Cryptophycin 52 | 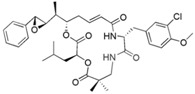 |
Apoptosis (40% at 250 μg/mL in LNCaP cells) | [27] |
| Lagunamide C | 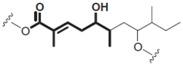 |
Cytotoxicity (IC50: 2.6 nM in PC-3 cells) | [28] | |
| Dolastatins | 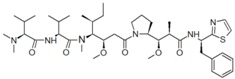 |
Cell cycle arrest (G2/M arrest in DU-145 cells) | [29] | |
| C-phycocyanin (C-PC) | 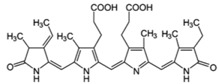 |
Inhibition of proliferation (30% inhibition at 500 μg/mL in LNCaP cells) | [30] | |
| Iejimalide B | 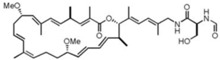 |
Cell cycle arrest (G0 / G1 arrest in LNCaP cells) | [31] | |
| Rhodophyta | Bromophycolide D | 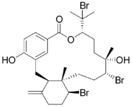 |
Cytotoxicity (IC50: 9.0 μM in PC-3 cells) | [32] |
| Chlorophyta | 14-keto-stypodiol diacetate (SDA) | 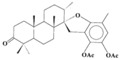 |
Cytotoxicity (IC50: 2.7 μM in DU145 cells) | [33] |
| Astaxanthin | 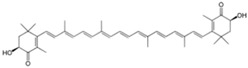 |
Inhibition of proliferation (38% inhibition at 0.01 μg/mL in LNCaP cells) | [34] | |
| Phaeophyta | Fucoidan | 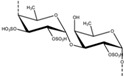 |
Apoptosis (15.2% at 10 μg/mL, 29.8% at 50 μg/mL, 39.3% at 100 μg/mL, and 45.1% at 200 μg/mL in PC3 cells) | [35,36] |
| Marine diatoms | Fucoxanthin | 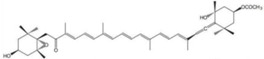 |
Inhibition of proliferation (50% inhibition at 2.5 μM in LNCaP cells) | [37,38] |
| Fucoxanthin, Fucoxanthinol, and Amarouciaxanthin A | 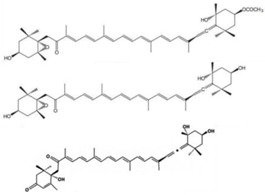 |
Cytotoxicity (IC50: 2.0–4.6 μM in PC-3 cells) | [39] | |
| Corals | Pachycladins A–E | 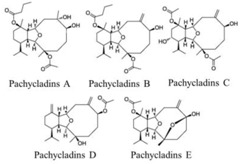 |
Inhibition of invasion (87% inhibition at 50 μM in PC-3 cells) | [40] |
| Metabolite 1 from Sarcophyton ehrenbergi, synthetic enantiomer (R)-1 | 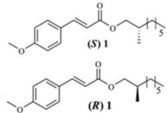 |
Cytotoxicity ((S)-1 (IC50: 161 mM in DU-145 cells); (R)-1 (IC50: 77.2 in DU145 cells) | [41] | |
| Holothurians | Frondoside A | 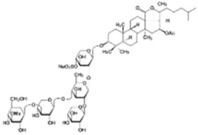 |
Cell cycle arrest (G2/M-phase at 0.5 µM in PC-3 cells) | [42] |
| 12-methyltetradecanoic acid | 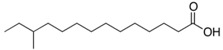 |
Cytotoxicity (IC50: 35.48 μg/mL in DU-145 cells, IC50: 20.45 μg/mL in PC-3 cells) | [43] |
