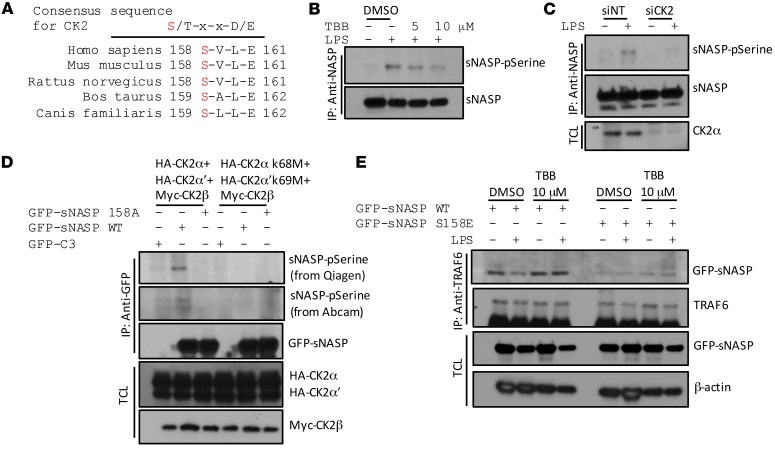
Figure 5. CK2 phosphorylates sNASP on serine 158.
(A) Alignment of sNASP sequence from multiple species revealed that serine 158 is highly conserved and contained in a motif recognized by CK2. X is any residue. (B) THP-1 cells stimulated with LPS in the absence (–) or presence (+) of TBB, an inhibitor of CK2, assessed by IB with antibody against phosphorylated serine (pSerine) or GFP after IP with anti-NASP. (C) THP-1 cells were stimulated with LPS in the presence of siNT or siCK2 and assessed by IB with antibody against phosphorylated serine (pSerine) or anti-GFP after IP with anti-NASP. TCL IB was done with anti-CK2α. (D) HEK293 cells transduced with GFP-tagged WT sNASP, S158A, or empty vector (C3) in the presence of Myc-tagged CK2β combined with HA-tagged CK2 catalytic subunits (CK2α and CK2α’) or CK2 kinase-dead mutants (CK2α K68M and CK2α’ K69M), assessed by IB with antibody against phosphorylated serine (pSerine) from Qiagen or Abcam or anti-GFP after IP with anti-GFP. TCL was IB with anti-HA or anti-Myc. (E) THP-1 cells transduced with GFP-tagged WT sNASP (WT) or S158A mutant in the absence (–) or presence (+) of TBB, assessed by IB with antibody against TRAF6 or GFP after IP with anti-TRAF6. TCL IB was done with anti-NASP and anti–β-actin. Data represent a minimum of 3 independent experiments.