Abstract
Introduction
There is concern about excessive bleeding when low-molecular-weight heparins (LMWHs) are used for venous thromboembolism (VTE) prophylaxis in renal dysfunction. Our objective was to evaluate whether LMWH VTE prophylaxis was safe and effective in critically ill patients with renal dysfunction by conducting a subgroup analysis of PROTECT, a randomized blinded trial.
Methods
We studied intensive care unit (ICU) patients with pre-ICU dialysis-dependent end-stage renal disease (ESRD; pre-specified subgroup; n = 118), or severe renal dysfunction at ICU admission (defined as ESRD or non-dialysis dependent with creatinine clearance [CrCl] <30 ml/min; post hoc subgroup; n = 590). We compared dalteparin, 5000 IU daily, with unfractionated heparin (UFH), 5000 IU twice daily, and considered outcomes of proximal leg deep vein thrombosis (DVT); pulmonary embolism (PE); any VTE; and major bleeding. Adjusted hazard ratios [HR] were calculated using Cox regression.
Results
In patients with ESRD, there was no significant difference in DVT (8.3% vs. 5.2%, p = 0.76), any VTE (10.0% vs. 6.9%; p = 0.39) or major bleeding (5.0% vs. 8.6%; p = 0.32) between UFH and dalteparin. In patients with severe renal dysfunction, there was no significant difference in any VTE (10.0% vs. 6.4%; p = 0.07) or major bleeding (8.9% vs. 11.0%; p = 0.66) but an increase in DVT with dalteparin (7.6% vs. 3.7%; p = 0.04). Interaction p-values for comparisons of HRs (ESRD versus not) were non-significant.
Conclusions
In critically ill patients with ESRD, or severe renal dysfunction, there was no significant difference in any VTE or major bleeding between UFH and dalteparin. Patients with severe renal dysfunction who received dalteparin had more proximal DVTs than those on UFH; this finding did not hold in patients with ESRD alone.
Introduction
Patients admitted to an intensive care unit (ICU) are at high risk for developing venous thromboembolism (VTE), which comprises deep vein thrombosis (DVT) and pulmonary embolism (PE). Despite prophylaxis with unfractionated heparin (UFH), 5% of patients develop proximal DVT, and this rate is higher among patients with septic shock [1, 2]. VTE prophylaxis with low-molecular-weight heparin (LMWH) is an appealing option in ICU patients due to administration ease (daily dosing), availability of pre-filled syringes (reducing the chance of medication error), and a lower incidence of heparin-induced thrombocytopenia than UFH [3, 4]. However, LMWH use in ICU patients may be concerning because, unlike UFH, these agents are cleared mainly by the kidney [5], and a high proportion of ICU patients will have acute or chronic renal dysfunction. Such patients also exhibit complex imbalances between procoagulant and anticoagulant systems related to nephrotic loss of natural anticoagulants, impaired fibrinolysis, administration of exogenous anticoagulants (i.e., during dialysis), and uremic platelet dysfunction [6].
Clinically important bioaccumulation occurs in patients with renal dysfunction who receive therapeutic doses of LMWHs (e.g., dalteparin 12000–18000 IU daily), which are typically used to treat acute VTE. These doses are avoided in patients with severe renal dysfunction, which we define as either creatinine clearance (CrCl) <30 mL/min without dialysis dependence, or dialysis-dependent end-stage renal disease (ESRD) [7, 8]. On the other hand, in such patients who receive prophylactic-dose LMWH (e.g., dalteparin 5000 IU daily), there is evidence from small observational studies of no bioaccumulation when such low-dose regimens are used for 7–10 days [9–13].
Few studies have evaluated LMWHs for VTE prophylaxis in ICU patients with severe renal dysfunction. In a pilot study of 19 ICU patients with renal impairment, including several patients needing acute hemodialysis, bioaccumulation was not observed with prophylactic LMWH (dalteparin, 5000 IU daily) [11]. Similarly, in a multicentre prospective cohort study of 138 ICU patients with an estimated CrCl <30 mL/min, no bioaccumulation occurred with prophylactic dalteparin [10]. Major bleeding occurred in 7% of these patients, but all had low trough anti-factor Xa levels (≤0.18 IU/mL). Moreover, none of these studies assessed bioaccumulation or clinical outcomes when LMWH was compared to VTE prophylaxis with UFH, which is dependent on non-renal mechanisms for clearance.
To further evaluate the premise that prophylactic LMWH should be avoided in patients with severe renal dysfunction, we conducted a subgroup analysis of PROTECT, a randomized trial comparing dalteparin (5000 IU daily) with UFH (5000 IU twice-daily) for VTE prophylaxis in critically ill patients [2]. Our primary pre-specified objective was to compare the efficacy (VTE) and safety (major bleeding) of dalteparin and UFH in patients with dialysis-dependent ESRD before ICU admission. Our secondary post hoc objective was to conduct the same comparisons in patients with a broader spectrum of renal impairment, encompassing those with dialysis-dependent ESRD or CrCl <30 mL/min without dialysis.
Materials and methods
Study design
The PROTECT study was a multicentre, randomized, blinded controlled trial comparing the LMWH dalteparin, 5000 IU daily, to UFH, 5000 IU twice-daily, for the prevention of VTE in critically ill ICU patients.[2] The primary outcome in PROTECT was proximal leg DVT; secondary outcomes included major bleeding, PE, and any VTE. The complete date range for participant recruitment and follow-up was May 2006 to June 2010. PROTECT was approved by research ethics boards of all participating hospitals (S1 Appendix).
We conducted a primary pre-specified subgroup analysis in patients with ESRD, defined by pre-ICU (baseline) dialysis dependence, in whom we postulated a priori based on our previous work [10] that LMWH would not be associated with higher rates of VTE or major bleeding than UFH. A secondary post hoc analysis was done in patients with severe renal dysfunction at ICU admission, defined as dialysis-dependent ESRD or non-dialysis-dependent but with an estimated CrCl <30 mL/min. We considered but rejected a secondary post hoc analysis in patients not on dialysis but with estimated CrCl <30 mL/min, reasoning that the larger group of patients with severe renal dysfunction was more relevant to clinicians. For these subgroup analyses, there was no sample size calculation at the time the main trial was designed.
Patients
Patients with the following inclusion criteria were enrolled: adults (age ≥18 years); body weight >45 kg; and expected ICU length of stay >72 hours. Exclusion criteria were major trauma; neurosurgery; orthopedic surgery; need for therapeutic anticoagulation; unfractionated heparin administration in the ICU for 3 days; contraindication to heparin or blood products; pregnancy; life-support limitation; or enrollment in a related trial. Research coordinators obtained written informed consent from all patients or their surrogates, as approved by research ethics boards of all participating hospitals. Patients were followed until the time of death in the hospital or discharge.
ESRD was defined as dependence on hemodialysis or peritoneal dialysis prior to ICU admission. Patients with severe renal dysfunction had either ESRD or CrCl <30 mL/min without dialysis dependence at the time of ICU admission, estimated by the Cockroft-Gault equation [14]. Patients with an initial CrCl <30 mL/min whose renal function improved during the ICU stay were retained within the severe renal dysfunction group, whereas patients with a CrCl ≥30 mL/min at the time of ICU admission who developed worsening renal function during the ICU stay were not included in the severe renal dysfunction group.
The subgroup analysis was designed by the authors and received approval from the PROTECT study Steering Committee. Funding for PROTECT was provided by the Canadian Institutes of Health Research, the Australian and New Zealand College of Anaesthetists Research Foundation, and the Heart and Stroke Foundation of Canada. Study drugs were provided by Pfizer and Eisai. Neither the funders nor the drug manufacturers played any role in the design or conduct of the trial or in the analysis or interpretation of the data. This trial is registered at ClinicalTrials.gov (identifier: NCT00182143).
Anticoagulant regimen
Using a centralized electronic system, local research pharmacists randomly assigned enrolled patients to receive either subcutaneous LMWH (dalteparin 5000 IU daily) or UFH (5000 IU twice daily). Randomization was stratified according to centre and type of admission (medical versus surgical) with the use of undisclosed variable block sizes in a 1:1 ratio. Research pharmacists prepared identical syringes for subcutaneous injection of either dalteparin daily plus placebo daily (for parallel group twice-daily injections), or of UFH twice daily for the duration of the ICU stay. Patients, family members, clinicians, research personnel, ultrasonographers, and outcome adjudicators were all unaware of study-group assignments.
Outcomes
For this study, the outcomes were proximal DVT, PE, any VTE, and major bleeding. Proximal DVT was defined as a new non-compressible vein segment of the popliteal or more proximal veins of the leg. All patients had routine screening bilateral leg venous ultrasound within 48 hours of study enrolment and twice weekly thereafter, as well as when clinically indicated. DVT diagnosed on the first screening ultrasound was considered prevalent DVT and was not included as a study outcome, whereas DVT detected subsequently was considered incident DVT and included as an outcome. Catheter-related DVT was classified as DVT according to its location (arm or leg). Routine screening for PE was not done. PE was defined as definite (characteristic intraluminal filling defect on chest computed tomography, a high probability ventilation–perfusion scan or autopsy finding), probable (high clinical suspicion and either no test results or non-diagnostic results on non-invasive testing), possible (clinical suspicion and a non-diagnostic test), or absent (negative tests). For this study, PE included definite, probable, or possible PE.
Major bleeding was defined as hemorrhage at a critical site (e.g., intracranial), necessitating a major therapeutic intervention (e.g., surgery), causing hemodynamic compromise, requiring 2 units of red-cell concentrates, or resulting in death. Minor bleeding was defined as bleeding that did not fulfill the criteria for major bleeding (e.g., heparin injection-site hematoma).
All VTE and major bleeding outcomes were independently adjudicated [2, 15, 16]. In formal calibration exercises, there was high concordance with respect to all outcomes [15, 16]. Thereafter, we randomly assigned each outcome to 2 adjudicators (or 4 adjudicators in the case of PE) who were unaware of randomized assignment and of one another’s assessments. Consensus was obtained for all outcomes with continued high levels of agreement throughout the trial.
Statistical analysis
Descriptive statistics (mean, standard deviation [SD]; median, inter-quartile range [IQR]; number, percentage) were used to summarize baseline characteristics. The incidences of VTE (proximal DVT, PE, any VTE) and major bleeding according to treatment allocation (dalteparin or UFH) were reported in patients with ESRD vs. no ESRD and, in a different analysis, in patients with severe renal dysfunction (ESRD or CrCl <30 mL/min without dialysis dependence) vs. no severe renal dysfunction (CrCl ≥30 mL/min).
Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) and p-values for the effect of dalteparin versus UFH on VTE and major bleeding outcomes in each of these subgroups were obtained from Cox regression analysis, stratified by centre and medical/surgical admission. For these analyses, all patients were included in a Cox regression model that included the effect of baseline ESRD (or ESRD or CrCl <30 mL/min without dialysis dependence in a second model) on VTE and bleeding outcomes plus its interaction with study drug (LMWH or UFH). Hazard ratios for VTE events were adjusted for APACHE II score, personal history of VTE, family history of VTE, and baseline inotrope/vasopressor use, whereas hazard ratios for bleeding events were adjusted for APACHE II score. Covariates in the adjusted models were selected a priori. The variables in the Cox regression models were verified to meet the assumption of proportional hazards. The plots of Martingale residuals and deviance residuals did not indicate any problems with model fit.
We interpreted (2-sided) p<0.05 as statistically significant. Statistical analyses were conducted using SAS (version 9.4; Cary, USA).
Results
Patients
Of the 6034 patients who met the enrollment criteria for PROTECT, 4574 were approached for consent (Fig 1). Consent was obtained for 3764 of these patients (82.3%). Consent was subsequently withdrawn for 18 patients. Of the 3746 patients in the intention-to-treat analysis, 1873 patients were assigned to receive dalteparin and 1873 to receive unfractionated heparin. No patients were lost to follow-up. This substudy focused on 118 patients with ESRD (mean age, 63 years; 39% female), 590 patients with ESRD or CrCl <30 mL/min (mean age, 69 years; 46% female; this group included the 118 patients with ESRD plus 472 patients not on dialysis with CrCl <30 mL/min), and 3089 patients with CrCl ≥30 mL/min. The median (IQR) duration of DVT prophylaxis was 7 (4–12) days in all patients. Additional patient characteristics are shown in Table 1.
Fig 1. CONSORT diagram: Patient flow through the study.
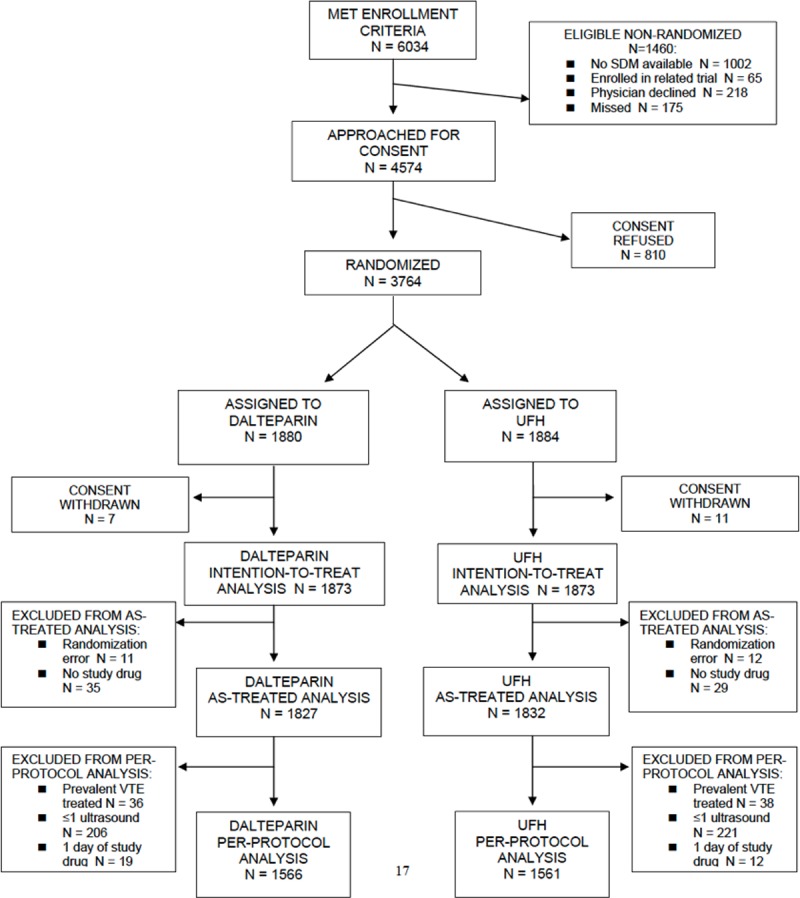
Table 1. Patient characteristics.
| ESRD (N = 118) | ESRD or CrCl <30 ml/min and no dialysis (N = 590) | CrCl ≥30 ml/min (N = 3089) | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 62.6 (14.1) | 68.6 (14.6) | 60.1 (16.5) |
| Female, N (%) | 46 (39.0) | 270 (45.8) | 1320 (42.7) |
| APACHE II score, mean (SD) | 27.3 (7.4) | 26.9 (7.3) | 20.5 (7.4) |
| BMI, mean (SD) | 28.1 (7.5) | 27.3 (6.7) | 28.5 (7.9) |
| Medical admission, N (%) | 94 (79.7) | 449 (76.1) | 2327 (75.3) |
| Personal history of VTE, N (%) | 8 (6.8) | 20 (3.4) | 99 (3.2) |
| Family history of VTE, N (%) | 1 (0.8) | 5 (0.8) | 51 (1.7) |
| History of malignancy, N (%) | 5 (4.2) | 21 (3.6) | 126 (4.1) |
| Admitting diagnosis, N (%) | |||
| Cardiovascular | 14 (11.9) | 65 (11.0) | 266 (8.6) |
| Respiratory | 31 (26.3) | 179 (30.3) | 1496 (48.4) |
| Gastrointestinal | 9 (7.6) | 86 (14.6) | 430 (13.9) |
| Renal | 13 (11.0) | 45 (7.6) | 20 (0.6) |
| Neurologic | 8 (6.8) | 17 (2.9) | 205 (6.6) |
| Sepsis | 31 (26.3) | 151 (25.6) | 398 (12.9) |
| Metabolic | 2 (1.7) | 20 (3.4) | 122 (3.9) |
| Other–medical | 2 (1.7) | 9 (1.5) | 54 (1.7) |
| Other–surgical | 8 (6.8) | 18 (3.1) | 98 (3.2) |
| Life support, N (%) | |||
| Mechanical ventilation | 94 (80.3) | 513 (87.1) | 2808 (90.9) |
| Vasopressors | 57 (48.7) | 349 (59.3) | 1305 (42.2) |
| Central venous catheterization | 108 (92.3) | 548 (93.0) | 2483 (80.4) |
Comparison of outcomes with dalteparin and UFH prophylaxis
In patients with ESRD (Table 2), there was no significant difference between those who received dalteparin and UFH in the incidence of proximal DVT (8.3% vs. 5.2%; HR 1.32, 95% CI 0.23–7.67, p = 0.76) or any VTE (10.0% vs. 6.9%; HR 2.08, 95% CI 0.39–11.17, p = 0.39). No PEs were observed in this group. There was no significant difference in major bleeds between patients who received dalteparin and UFH (5.0% vs. 8.6%; HR 0.47, 95% CI 0.11–2.08, p = 0.32).
Table 2. Subgroup analyses based on baseline ESRD (pre-ICU chronic dialysis).
| ESRD | Total (N = 118) | Dalteparin (N = 60) | UFH (N = 58) | Hazard Ratio† (95% CI) | p-value |
| Proximal DVT, N (%) | 8 (6.8) | 5 (8.3) | 3 (5.2) | 1.32 (0.23–7.67) | 0.76 |
| VTE, N (%) | 10 (8.5) | 6 (10.0) | 4 (6.9) | 2.08 (0.39–11.17) | 0.39 |
| PE, N (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - |
| Major bleed, N (%) | 8 (6.8) | 3 (5.0) | 5 (8.6) | 0.47 (0.11–2.08) | 0.32 |
| No ESRD | Total (N = 3609) | Dalteparin (N = 1805) | UFH (N = 1804) | Hazard Ratio† (95% CI) | p-value |
| Proximal DVT, N (%) | 197 (5.5) | 91 (5.0) | 106 (5.9) | 0.91 (0.67–1.22) | 0.52 |
| VTE, N (%) | 330 (9.1) | 148 (8.2) | 182 (10.1) | 0.85 (0.67–1.08) | 0.18 |
| PE, N (%) | 67 (1.9) | 24 (1.3) | 43 (2.4) | 0.50 (0.29–0.86) | 0.01 |
| Major bleed, N (%) | 200 (5.5) | 100 (5.5) | 100 (5.5) | 1.03 (0.77–1.38) | 0.83 |
Abbreviations: DVT, deep venous thrombosis; ESRD, end-stage renal disease; PE, pulmonary embolism; UFH, unfractionated heparin; VTE, venous thromboembolism.
† Hazard ratios obtained from Cox models stratified by centre and medical/surgical admission. Each model contains a term for group (baseline ESRD vs. not), treatment group (dalteparin vs. UFH), and an interaction term. For thrombotic events (proximal DVT, VTE, PE), hazard ratios are adjusted for APACHE II score, personal history of VTE, family history of VTE, and baseline inotrope/vasopressor use. For major bleed events, hazard ratios are adjusted for APACHE II score. Interaction p-values between adjusted hazard ratios for patients with ESRD and patients without ESRD were not significant (p = 0.68 for proximal DVT, p = 0.30 for VTE, and p = 0.31 for major bleeding).
In patients with severe renal dysfunction (ESRD or CrCl <30 mL/min without dialysis dependence, Table 3), there were significantly more proximal DVTs in patients who received dalteparin than UFH (7.6% vs. 3.7%; HR 0.47, 95% CI 0.11–2.08, p = 0.04). There was a non-significant increase in any VTE in the dalteparin group (10.0% vs. 6.4%; HR 1.87, 95% CI 0.96–3.63, p = 0.07). There was no significant difference in PEs (0.7% vs. 1.0%; HR 0.37, 95% CI 0.04–3.68, p = 0.39) and no significant difference in major bleeds (8.9% vs. 11.0%, HR 0.89, 95% CI 0.51–1.53, p = 0.66) between patients who received dalteparin and UFH.
Table 3. Subgroup analyses based on baseline severe renal dysfunction (ESRD or CrCl <30 mL/min without dialysis dependence).
| Severe renal dysfunction | Total (N = 590) | Dalteparin (N = 291) | UFH (N = 299) | Adjusted Hazard Ratio† (95% CI) | p-value |
| Proximal DVT, N (%) | 33 (5.6) | 22 (7.6) | 11 (3.7) | 2.34 (1.03–5.34) | 0.04 |
| Any VTE, N (%) | 48 (8.1) | 29 (10.0) | 19 (6.4) | 1.87 (0.96–3.63) | 0.07 |
| Any PE, N (%) | 5 (0.8) | 2 (0.7) | 3 (1.0) | 0.37 (0.04–3.68) | 0.39 |
| Major bleed, N (%) | 59 (10.0) | 26 (8.9) | 33 (11.0) | 0.89 (0.51–1.53) | 0.66 |
| No severe renal dysfunction | Total (N = 3089) | Dalteparin (N = 1550) | UFH (N = 1539) | Adjusted Hazard Ratio† (95% CI) | p-value |
| Proximal DVT, N (%) | 171 (5.5) | 73 (4.7) | 98 (6.4) | 0.78 (0.56–1.08) | 0.13 |
| Any VTE, N (%) | 290 (9.4) | 124 (8.0) | 166 (10.8) | 0.77 (0.60–0.99) | 0.04 |
| Any PE, N (%) | 61 (2.0) | 22 (1.4) | 39 (2.5) | 0.50 (0.29–0.88) | 0.02 |
| Major bleed, N (%) | 145 (4.7) | 75 (4.8) | 70 (4.5) | 1.08 (0.76–1.52) | 0.68 |
Abbreviations: DVT, deep venous thrombosis; ESRD, end-stage renal disease (defined as dialysis dependence before ICU admission); PE, pulmonary embolism; UFH, unfractionated heparin; VTE, venous thromboembolism.
† Hazard ratios obtained from Cox models stratified by centre and medical/surgical admission. Each model contains a term for group (severe renal dysfunction vs. not), treatment group (dalteparin vs. UFH), and an interaction term. For thrombotic events (proximal DVT, VTE, PE), hazard ratios are adjusted for APACHE II score, personal history of VTE, family history of VTE, and baseline inotrope/vasopressor use. For major bleed events, hazard ratios are adjusted for APACHE II score. Interaction p-values between adjusted hazard ratios for patients with baseline renal dysfunction vs. not were significant for proximal DVT (p = 0.02) and VTE (p = 0.01), but not for PE (p = 0.80) or major bleeding (p = 0.56).
When VTE and major bleeding outcomes were analyzed in subgroups defined by ESRD, the interaction p-values for the difference between all HRs were non-significant (p>0.05) (Table 2). When similar analyses were done for patients with severe renal dysfunction (ESRD or CrCl<30 mL/min without dialysis dependence), interaction p-values for the difference between the HRs for proximal DVT (p = 0.02) and VTE (p = 0.01) were significant. Dalteparin prophylaxis was associated with a higher risk for proximal DVT and any VTE in patients with severe renal dysfunction, which was statistically significant for proximal DVT, while dalteparin was associated with lower risk for these events in patients without severe renal dysfunction (i.e. patients with CrCl >30 mL/min), which was statistically significant for any VTE. All other interaction p-values were non-significant (p>0.05) (Table 3).
In a further exploratory analysis, we assessed possible imbalances in VTE risk factors (not adjusted for in the regression analysis) among patients with severe renal dysfunction who received dalteparin and UFH. We found no differences between treatment groups in mean body mass index (27.4 kg/m2 vs. 27.2 kg/m2), or in the proportion of patients with a femoral vein catheter (19.7% vs. 18.4%) or any central venous catheter (94.8% vs. 96.7%).
Discussion
In critically ill patients with dialysis-dependent ESRD at baseline, an a priori subgroup analysis of data from the PROTECT trial showed no significant difference in proximal DVT, any VTE or major bleeding between VTE prophylaxis with dalteparin, 5000 IU daily, compared to UFH, 5000 IU twice-daily. In a post hoc analysis of patients with severe renal dysfunction, defined as baseline ESRD or CrCl <30 mL/min without dialysis dependence, there was no significant difference in major bleeding between the dalteparin and UFH groups. However, patients who received dalteparin had a non-significant increased risk of any VTE and a significant increased risk of proximal DVT.
The overall results of the PROTECT trial, which includes the patients presented in this report, showed that dalteparin, compared with UFH, had no significant difference in major bleeding (5.5% vs. 5.6%) and proximal DVT (5.1% vs. 5.8%) but a significantly lower risk of PE (1.3% vs. 2.3%) when used for VTE prophylaxis in critically ill patients[2]. The results of the present subgroup analysis are consistent with the overall findings for the safety outcome of major bleeding and support the premise that low-dose dalteparin, when administered over a 7–10 day period, appears safe for use in patients with ESRD or CrCl <30 mL/min without dialysis dependence.
However, there are some discrepant findings for the efficacy outcomes that require careful interpretation. Whereas rates of proximal DVT were not significantly different in the dalteparin and UFH groups with ESRD (8.3% vs. 5.2%, p = 0.76), there was a significantly higher rate of proximal DVT in the dalteparin than UFH group when a larger population with severe renal dysfunction (7.6% vs. 3.7%, p = 0.04) was studied. Acknowledging that the latter was a post hoc comparison, there are three possible explanations for these discrepant findings. First, it is possible that the findings are true and are explained by a more effective anticoagulant effect of UFH over dalteparin in patients with ESRD or CrCl <30 mL/min. Potentially supporting this premise is the observation that major bleeding events were numerically higher in the UFH than dalteparin group (11.0% vs. 8.9%, p = 0.76); however, the risks were not statistically distinguishable. Although one may therefore speculate that heparin has a greater overall anticoagulant effect compared with dalteparin, this premise is unlikely, as low-dose UFH is cleared mainly by non-renal mechanisms [5]. At the doses used in the PROTECT trial, it would be unlikely for UFH to bioaccumulate more than dalteparin, leading to more bleeds and fewer DVT events. Second, it is possible that the findings are true and are explained by a more persistent antithrombotic effect of the twice-daily dosing of UFH than the once-daily dosing of dalteparin, which may have more impact among patients with severe renal dysfunction compared to other patients. Supporting this premise is that in patients without severe renal dysfunction (i.e. those with CrCl ≥30 ml/min), rates of proximal DVT were numerically lower in the dalteparin than UFH group (4.7% vs. 6.4%, p = 0.13). Finally, the finding of more proximal DVT in the dalteparin than UFH group may be spurious and due to the play of chance. In support of this premise, the subgroup with severe renal dysfunction represented only 16% of the total PROTECT study population. Moreover, the effect of more proximal DVT in the dalteparin group was not evident in the closely related outcome of PE.
On balance, further study is required before any definitive conclusions can be made about the efficacy of dalteparin compared with UFH for the prevention of proximal DVT in patients with severe renal dysfunction. Similarly, further study is required to explore the numerically increased (albeit statistically insignificant) major bleeding events in the UFH group. Renal dysfunction results in complex hemostatic imbalances, including decreased platelet aggregation and impaired platelet adhesion (partly due to impaired function of platelet membrane glycoprotein IIb/IIIa) [17, 18]. UFH and LMWH have different effects on platelet receptor activation and platelet aggregation in vitro, so it is possible that their in vivo actions in individuals with impaired primary hemostasis also differ, leading to more bleeding with UFH [19].
We acknowledge additional potential limitations of our study. First, the number of patients studied, especially those with ESRD, was relatively small compared to the entire study sample. As a result, confidence intervals for effects that were statistically negative were wide, and effect estimates may change in future trials with more patients and events. Although this is the largest study, to our knowledge, of patients with ESRD who received LMWH prophylaxis, additional research is warranted. Second, the mean duration of anticoagulant prophylaxis was 7 days, so our findings may not be applicable to patients who receive a considerably longer duration of prophylaxis. Third, the study did not consider the efficacy and safety of LMWHs other than dalteparin.
Our findings are relevant to clinical practice as they further challenge the premise that LMWHs should be avoided in patients with renal dysfunction because of presumed bioaccumulation and the potential for an increase in bleeding. We acknowledge the inconsistent finding for the outcome of DVT in the groups with ESRD compared to the larger subgroup of severe renal dysfunction (ESRD or CrCl<30 mL/min without dialysis dependence); we believe that this finding should be interpreted with caution and warrants further study.
In critically ill patients with ESRD and those with severe renal dysfunction more broadly defined, there was no significant difference in any VTE or major bleeding between UFH and dalteparin. Patients with ESRD or CrCl <30 mL/min without dialysis dependence who received dalteparin had more proximal DVTs than those who received UFH; this finding did not hold in patients with ESRD alone. This discrepant finding merits further investigation.
Supporting information
(DOCX)
(PDF)
(PDF)
Acknowledgments
We thank the patients, families, research coordinators, study pharmacists, bedside nurses, intensivists, ultrasonographers, and radiologists who participated. The trial was designed by the PROTECT (Prophylaxis for ThromboEmbolism in Critical Care Trial) Steering Committee, PROTECT Investigators and the Canadian Critical Care Trials Group. PROTECT was supported by the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Research Coordinators collected data.
PROTECT Collaborators
Lead Author
• Dr. Deborah Cook, St Joseph’s Healthcare, Hamilton, Canada.
• Email: debcook@mcmaster.ca
Canadian Investigators
• Drs. Rick Hall & Graeme Rocker, Lisa Julien, Debbie Wright, Caroline Roy, Judy Theriault, Sue Pleasance; Pharmacy Technician Debi Snow and Shannon Herbert; Capital Health QEII Health Science Center, Halifax
• Dr. Maureen Meade, Lori Hand; Pharmacy Technician Maya Biljan; Hamilton Health Sciences, Hamilton General Hospital, Hamilton
• Dr. Andreas Freitag, Christine Wynne, Mark Duffett, Michelle Kho, Nicole Zytaruk; Pharmacy Technician Karen Currie; Hamilton Health Sciences, McMaster Hospital, Hamilton
• Dr. John Granton, Andrea Matte, Paulina Farias, Leslie Chu, Nancy Brockest, Stephanie Go, Margaret McGrath-Chong, Madison Dennis, Marc Lipkus; Pharmacist Jie Ming & Laura Arus Pampin; University Health Network, Toronto General Hospital, Toronto
• Drs. Stephan Langevin, François Lauzier, & Alexis Turgeon, Anik Rioux, Martine Blais, Maxime Beauparlant, Julie Asselin, Caroline Roy, Chantal Gagne; Pharmacist Tuong Vi-Tran; Hôpital de l'Enfant-Jésus, Québec City
• Dr. Germain Poirier, Isabelle Neas, Sandrine Spearson; Pharmacist Betty Ton; Charles LeMoyne Hospital, Montreal
• Drs. Lauralyn McIntyre & Paul Hebert, Irene Watpool, Tracy McArdle, Paule Marchand, Claude Gaudert, Carson Davidson, Pharmacist Ane-Marie Dugal and Susan Fetzer; Ottawa Hospital, General Campus, Ottawa
• Dr. Joe Pagliarello, Mary-Jo Lewis, Erin Murphy, Julia Foxall; Pharmacist Sherry Weir; Ottawa Hospital Civic Campus, Ottawa
• Dr. Yoanna Skrobik, Johanne Harvey, Stefania Chitu; Pharmacist Marceline Quach and Linda Pinet; Maisonneuve Rosemont Hospital, Montréal
• Dr. Martin Albert, Carole Sirois, Carole Nadon, Stephanie Dolle, Audrey-Anne Gosselin, Patrice Deroy; Pharmacist Anne Julie Frenette and David Williamson; Hôpital du Sacré-Coeur de Montréal, Montreal
• Dr. Sangeeta Mehta, Cheryl Ethier, Sam Tirgari, Lindsay Steinberg, Rod McDonald, Vidhya Sivanantham, Kristofer Bandayrel, Friederike Quittnat Pelletier, Marnie Kramer-Kile, Maedean Brown, Scott Kim; Pharmacist Holly Leung; Mount Sinai Hospital, Toronto
• Dr. Robert Fowler, Nicole Marinoff, Karen Code, Boris Bojilov, Derek Parsotam; Pharmacist John Iazzetta; Sunnybrook Health Sciences Centre, Toronto
• Dr. John Marshall, Orla Smith, Beth Fry, Kerri Porretta, Yoon Lee, Jeanna Morrissey, Victoria Wen; Pharmacy Technicians Laura Parsons and Ann Kosinski; St. Michael’s Hospital, Toronto
• Dr. John Muscedere, Susan Fleury, Nicole Godfrey, Sharlene Hammond, Elizabeth Mann, Monica Myers, Amber Robinson; Pharmacist Chris Grey; Kingston General Hospital, Kingston
• Drs. Sean Keenan & Steven Reynolds, Miroslav Svetik, Mary Van Osch; Pharmacist Anne-Marie Liberman; Royal Columbian Hospital, Westminster
• Dr. Dean Chittock, Maureen Gardner, Susan Logie, Denise Foster, Roger Autio, Dara Davies, Pia Ganz, Laurie Smith; Pharmacist Judy Yip; Vancouver General Hospital, Vancouver
• Dr. Peter Dodek, BettyJean Ashley, Sheilagh Mans; Pharmacist Mara Pavan; St. Paul’s Hospital, Vancouver
• Dr. Chip Doig, Linda Knox, Crystal Wilson, Kevin Champagne; Pharmacist Angela Kayall Peters: Calgary University, Calgary
• Dr. Niall Ferguson, Andrea Matte, James Stevenson, Joel Elman, Madison Dennis; Pharmacist Jenn Tung; University Health Network, Toronto Western Hospital, Toronto
• Dr. Jim Kutsogiannis, Patrica Thompson, Norine Whalen; Pharmacist Liz Helboe; Royal Alexandra Hospital, Edmonton
• Dr. François Lellouche, Marie-Claude Ferland, Patrick Dussault, Caroline Jacob, Marie-Eve Morneau, Nancy Laberge; Pharmacist Nathalie Chateauvert; Laval Hospital, Quebec City
• Dr. Tim Karachi, Andrea Tkaczyk; Pharmacy Technician Diane Lourenco; Hamilton Health Sciences, Henderson Hospital, Hamilton
• Dr. Michael Jacka, Marleen Irwin, Carmen Chan, Leeca Sonnema, Kelly Marsh, Jennifer Maurer, Tamara Kreidl, Candice Varden, Carey Kinjerski; Pharmacist Noelle Carey; University of Alberta, Edmonton
• Dr. Kosar Khwaja, Laura Banici, Carole Sirois, Lena Havell; Pharmacist Gilbert Matte and Kathleen Normandin; Montréal General Hospital and Royal Victoria Hospital, Montréal
• Dr. Gordon Wood, Fiona Auld, Leslie Atkins; Pharmacist John Foster-Coull; Vancouver Island Health Authority, Vancouver
• Drs. Olivier Lesur & François Lamontagne, Sandra Proulx: Pharmacist Sylvie Cloutier: Sherbrooke University Hospital and Centre de Recherche Clinique Étienne-Le Bel, Sherbrooke
• Drs. Gerald Hollinger & Vasanti Shende, Vanessa Belcastro; Pharmacist Jane Martin; Guelph General Hospital, Guelph
• Dr. Bill Plaxton, Anders Foss; Pharmacist Colleen Cameron; Grand River Hospital, Kitchener
• Drs. Bojan Paunovic and Kim Wiebe, Nicole Marten; Pharmacist Denise Sawatzky: St Boniface Hospital, Winnipeg
• Dr. Jonathan Eisenstat, Tammy Doerle; Pharmacist Linda Skinner; Lakeridge Health, Oshawa
• Drs. Steven Reynolds & Sean Keenan, Sheilagh Mans; Pharmacist Ray Jang; Surrey Memorial Hospital, Surrey
• Dr. Michael Sharpe, Mona Madady; Pharmacist Chandika Mankanjee; London Health Sciences Centre, London
• Dr. Deborah Cook, Ellen McDonald, Andrea Tkaczyk, France Clarke; Pharmacist Christine Wallace; St Joseph’s Healthcare, Hamilton
Australian Investigators
• Drs. Jamie Cooper & Andrew Davies, Shirley Vallance, Cindy Weatherburn, Jasmin Board, Lucinda Gabriel; Pharmacist Anne Mak and Sook Wern Chua; Alfred Hospital, Melbourne
• Drs. Simon Finfer & Naresh Ramakrishnan(deceased), Simon Bird, Julie Potter, Anne O’Connor, Susan Ankers; Pharmacist Maggie Gibson; Royal North Shore Hospital, Sydney
• Dr. Jack Cade, Deborah Barge, Tania Caf, Belinda Howe; Pharmacist Robyn Ingram; Royal Melbourne Hospital, Melbourne
• Dr. Rinaldo Bellomo, Glenn Eastwood, Leah Peck, Donna Goldsmith, Kim O’Sullivan; Lead Pharmacist Dr Michael Ching, Jean Schmidt, Mei Ho & Bailey Lim; Austin Hospital, Melbourne
• Drs. David Ernest, Sam Radford, Ann Whitfield & Anthony Cross, Suzanne Eliott, Jaspreet Sidhu, Belinda Howe, Inga Mercer, Angela Hamilton (deceased); Pharmacist Paula Lee; Box Hill Hospital, Melbourne
• Dr. John Botha, Jodi Vuat, Sharon Allsop, Nina Fowler; Pharmacist Chui Yap; Frankston Hospital, Frankston
• Drs. Tim Crozier, Jonathan Barrett & Chris Wright, Pauline Galt, Carly Culhane, Rebecca Ioannidis, Sue Burton, Marnie Reily, Cyveen Weeraratna; Pharmacist Helen Koop; Monash Medical Centre, Melbourne
• Drs. Ian Seppelt, Leonie Weisbrodt, Robyn Bond; Pharmacist Stella Suen and Jason Trinh; Nepean Hospital, Sydney
• Dr. David Evans, Justine Rivett, Stephanie O’Connor, Alex Poole; Pharmacist Peter Slobodian; Royal Adelaide Hospital, Adelaide
• Dr. Clive Woolfe, Dorrilyn Rajbhandari, Caitlin Rees; Pharmacist Justine Hay; Royal Prince Alfred Hospital, Camperdown
• Drs. John Edington & Jason Fletcher, Julie Smith, Catherine Boschert; Pharmacist Richard Summers; Bendigo Health Care, Bendigo
• Dr Graham Reece, Treena Sara, Kiran Nand; Pharmacist Rabsima Ibrahim; Blacktown Hospital, Blacktown
• Dr. Andrew Bersten, Alex Gallus, Elisha Matheson, Margie O’Callaghan; Pharmacist Kelly Woolley; Flinders Medical Center, Adelaide
• Dr. Neil Orford, Tania Elderkin, Melissa Fraser, Allison Bone, Tania Salerno, Anne Kinmonth; Pharmacist Paul Muir; Barwon Health, Geelong Hospital, Geelong
• Dr. Subhash Arora, Bridget O’Bree, Katherine Shepherd; Pharmacist Kerry Gray, Tu Vinh; Dandenong Hospital, Dandenong
• Drs. Alan Davey–Quinn & Martin Sterba, Bronwyn Ruth Johnson, Renee Xu, Francisco Hill; Pharmacist Julie Thompson; Wollongong Hospital, Wollongong
• Dr. Rajaram Ramadoss, Josette Wood; Pharmacist Eric Tah Wai Yap; Lyell McEwin Hospital, Adelaide
Brazilian Investigators
• Dr. Marcelo Garcia da Rocha (Co-Lead), Andréa Kramer, Martha Hädrich; Pharmacist Patrícia Soares, Fernando Frosi; Santa Casa Hospital, Porto Allegre
• Dr. Nilton Brandao & Cassiano Teixeira & Cíntia Roehrig, Juliana Zeni; Pharmacist Daniel Panizzi; Moinhos de Vento Hospital, Porto Alegre
• Drs. Suzanna Alves & Rubens Costa Filho, Renato Correa & Plínio N. Gomes; Pharmacist Marcia Caneca; Graziela Silva; Pró Cardíaco Hospital, Rio de Janeiro
• Drs. Otavio Berwanger (Co-Lead) & Edson Romano, Anna Maria Buehler; Pharmacist Marcelo Murad & Paulo Buononato, Hospital Coracao Research Institute HCor, São Paulo
• Drs. Helio Penna Guimarães & Renato D Lopes, Adriano Truffa, Rosana Nakamura, Lillian Mazza Barbosa; Pharmacist: Rosana Suemi Nakamura, Hospital São Paulo, São Paulo
Saudi Arabian Investigators
• Dr. Ismael Qushmaq (Lead), Jean Brennick, Sawsan Bassi; Pharmacist Amnah Mukhtar, Majdah Al-Ghamdi & Amer Soliman; King Faisal Specialist Hospital and Research Center, Jeddah
• Dr. Mohammed Alsultan & Yaseen Arabi, Riette Brits; Pharmacist Antoine Cherfan; King Abdulaziz Medical City Hospital, Riyadh
• Dr. Jamal Alhashemi, Sanaa Shalabi; Pharmacist Randa Ainosah; King Abdulaziz University Hospital, Jeddah
• Drs. Yasser Mandourah & Nadeem Shaikh; Pharmacist Shatha Shosho; Riyadh Military Hospital, Riyadh
• Drs. Manal Al-Hazmi & M. Ali Al-Azem, Trevor Wyngaard; Pharmacist Yahya Moustafa; King Fahad Medical City Hospital, Riyadh
United States Investigators
• Dr. James Klinger, Barbara Smithson; Pharmacist Andrea Monckeberg; Rhode Island Hospital, Providence
• Dr. Nicholas E Vlahakis (Lead), Laurie Meade; Pharmacist Debbie Bauer; Mayo Clinic, Rochester
• Dr. Michael Cox, Jackie O’Brien, Catherine Krause; Pharmacist Sandra Ahearn; St John’s Mercy Medical Center, St Louis
• Drs. Joseph Nates & Sajid Haque, Deidre Mooring, Rose Erfe, Paula Nickerson; Pharmacist Kim McConnell; UTMD Anderson Cancer Center, Houston
United Kingdom Investigators
• Drs. Marlies Ostermann (Lead) & David Treacher, Tony Sherry, John Smith, Barnaby Sanderson, Josephine Ng, John Brooks, Ling Lim, Katie Lei; Pharmacists Paul Tunstell, Dr Cathy McKenzie, Francesco Cicirello; King's College London, Guy's & St Thomas’ Hospital, London
Radiology Consultants: Dr. David Schiff, St Josephs Healthcare, Hamilton, Canada, Dr. Allan Moody, Sunnybrook Hospital, Toronto, Canada
Ultrasound Technology Consultants: Jennifer McDonald (Canada), St Josephs Healthcare Hamilton, Hamilton, Judy Willis (Australia), The Alfred, Melbourne
Pharmacy Consultant: Gita Sobhi, Hamilton Health Science Center, Hamilton
PROTECT Steering Committee: Deborah Cook, Mark Crowther, Maureen Meade, Gordon Guyatt, Stephen Walter, Bill Geerts, Jamie Cooper (Australia), Ismael Qushmaq (Saudi Arabia), Marcelo Rocha, Otavio Berwanger (Brazil), Theodore Warkentin, Nicole Zytaruk (Project Manager, Canada), Shirley Vallance (Project Manager, Australia), Diane Heels-Ansdell (Biostatistician)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the Canadian Institute Health Research (#MCT78568), Heart & Stroke Foundation of Canada (#T6157, #T6950, #NA6186) and the Australian and New Zealand College of Anesthetists Research Foundation (#07/23). Pfizer Canada provided dalteparin for Canadian centers; Eisai, Inc, provided dalteparin for centers in the United States. None of these groups played a role in the design, conduct, analysis, interpretation or write-up of this trial. M Crowther is a Career Investigator of the Heart and Stroke Foundation of Canada, and is the Leo Pharma Chair in Thromboembolism Research at McMaster University. M Meade is a Mentor of the Canadian Institutes of Health Research. DJ Cooper is a National Health Medical Research Council Practitioner Fellow. D Cook is a Research Chair of the Canadian Institutes of Health Research. M Walsh is supported by a New Investigator Award from the Canadian Institutes of Health Research.
References
- 1.Kaplan D, Casper TC, Elliott CG, Men S, Pendleton RC, Kraiss LW, et al. VTE Incidence and Risk Factors in Patients With Severe Sepsis and Septic Shock. Chest. 2015;148(5):1224–30. doi: D—NLM: PMC4631038 [Available on 11/01/16] EDAT- 2015/06/26 06:00 MHDA- 2016/02/19 06:00 CRDT- 2015/06/26 06:00 AID - S0012-3692(15)50233-X [pii] AID—doi: 10.1378/chest.15-0287 [doi] PST—ppublish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Protect Investigators for the Canadian Critical Care Trials Group, The Australian New Zealand Intensive Care Society Clinical Trials Group, Cook D, Meade M, Guyatt G, Walter S, et al. Dalteparin versus unfractionated heparin in critically ill patients. The New England journal of medicine. 2011;364(14):1305–14. Epub 2011/03/23. doi: 10.1056/NEJMoa1014475 . [DOI] [PubMed] [Google Scholar]
- 3.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl E, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–226S. doi: D—NLM: PMC3278052 EDAT- 2012/02/15 06:00 MHDA- 2012/04/14 06:00 CRDT- 2012/02/09 06:00 AID - S0012-3692(12)60124-X [pii] AID—doi: 10.1378/chest.11-2296 [doi] PST—ppublish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e495S–530S. Epub 2012/02/15. doi: 10.1378/chest.11-2303 ; PubMed Central PMCID: PMCPMC3278058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boneu B, Caranobe C, Sie P. Pharmacokinetics of heparin and low molecular weight heparin. Bailliere's Clin Haematol. 1990;3:531–44. [DOI] [PubMed] [Google Scholar]
- 6.Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Seminars in thrombosis and hemostasis. 2010;36:34–40. doi: 10.1055/s-0030-1248722 [DOI] [PubMed] [Google Scholar]
- 7.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315–52. doi: 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 8.Lim W, Dentali F, Eikelboom JW, Crowther MA. Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal dysfunction. Annals of internal medicine. 2006;144(9):673–84. Epub 2006/05/04. . [DOI] [PubMed] [Google Scholar]
- 9.Bauersachs R, Schellong SM, Haas S, Tebbe U, Gerlach HE, Abletshauser C, et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal dysfunction. Thrombosis and haemostasis. 2011;105(6):981–8. Epub 2011/04/21. doi: 10.1160/TH10-09-0614 . [DOI] [PubMed] [Google Scholar]
- 10.Douketis J, Cook D, Meade M, Guyatt G, Geerts W, Skrobik Y, et al. Prophylaxis against deep vein thrombosis in critically ill patients with severe renal dysfunction with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the DIRECT study. Archives of internal medicine. 2008;168(16):1805–12. Epub 2008/09/10. doi: 10.1001/archinte.168.16.1805 . [DOI] [PubMed] [Google Scholar]
- 11.Rabbat CG, Cook DJ, Crowther MA, McDonald E, Clarke F, Meade MO, et al. Dalteparin thromboprophylaxis for critically ill medical-surgical patients with renal dysfunction. Journal of critical care. 2005;20(4):357–63. Epub 2005/11/29. doi: 10.1016/j.jcrc.2005.09.009 . [DOI] [PubMed] [Google Scholar]
- 12.Schmid P, Brodmann D, Fischer AG, Wuillemin WA. Study of bioaccumulation of dalteparin at a prophylactic dose in patients with various degrees of impaired renal function. Journal of thrombosis and haemostasis: JTH. 2009;7(4):552–8. Epub 2009/01/30. doi: 10.1111/j.1538-7836.2009.03292.x . [DOI] [PubMed] [Google Scholar]
- 13.Schmid P, Brodmann D, Fischer AG, Wuillemin WA. Prospective observational cohort study of bioaccumulation of dalteparin at a prophylactic dose in patients with peritoneal dialysis. Journal of thrombosis and haemostasis: JTH. 2010;8(4):850–2. Epub 2010/01/22. doi: 10.1111/j.1538-7836.2010.03749.x . [DOI] [PubMed] [Google Scholar]
- 14.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. Epub 1976/01/01. doi: 10.1159/000180580 . [DOI] [PubMed] [Google Scholar]
- 15.Zytaruk N, Meade M, Mehta S, Hall R, Southon J, Heels-Ansdell D, et al. Adjudication Calibration for Pulmonary Embolism in a Thromboprophylaxis Trial [abstract]. Am J Resp Crit Care Med. 2009;179:A1582. [Google Scholar]
- 16.Arnold DM, Lauzier F, Rabbat C, Zytaruk N, Barlow Cash B, Clarke F, et al. Adjudication of bleeding outcomes in an international thromboprophylaxis trial in critical illness. Thrombosis research. 2013;131(3):204–9. doi: 10.1016/j.thromres.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 17.Benigni A, Boccardo P, Galbusera M, Monteagudo J, De Marco L, Remuzzi G, et al. Reversible activation defect of the platelet glycoprotein IIb-IIIa complex in patients with uremia. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1993;22(5):668–76. Epub 1993/11/01. . [DOI] [PubMed] [Google Scholar]
- 18.Gawaz MP, Dobos G, Spath M, Schollmeyer P, Gurland HJ, Mujais SK. Impaired function of platelet membrane glycoprotein IIb-IIIa in end-stage renal disease. J Am Soc Nephrol. 1994;5(1):36–46. Epub 1994/07/01. . [DOI] [PubMed] [Google Scholar]
- 19.Xiao Z, Theroux P. Platelet activation with unfractionated heparin at therapeutic concentrations and comparisons with a low-molecular-weight heparin and with a direct thrombin inhibitor. Circulation. 1998;97(3):251–6. Epub 1998/02/14. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


