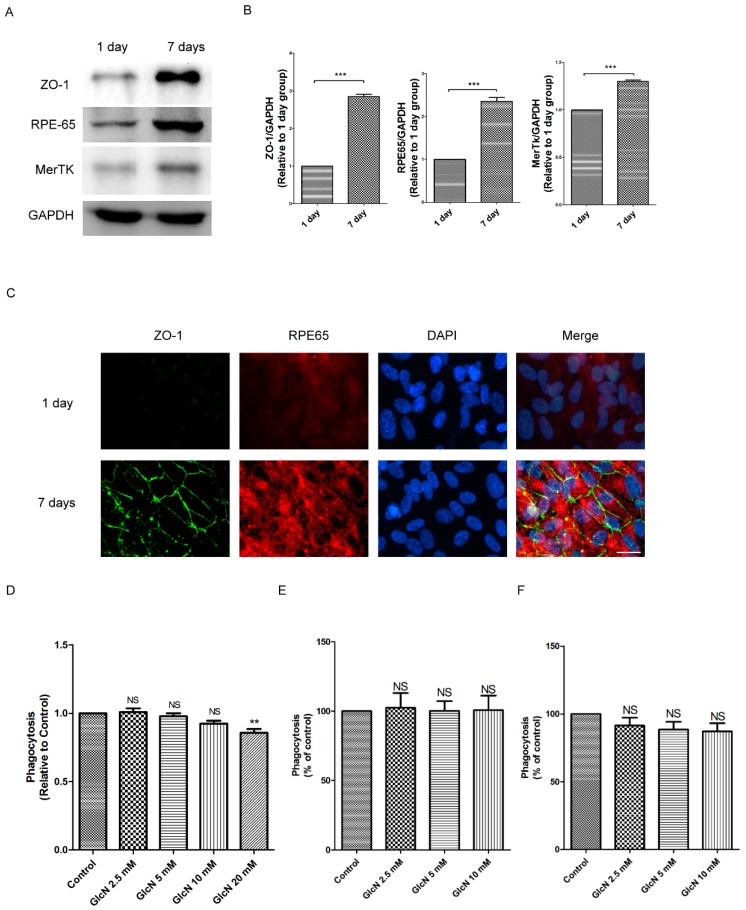
Figure 1.
Expression of ZO-1, RPE65, and MerTK protein after one- and seven-day cultures in ARPE-19 cells. (A) Western blot analysis detecting the protein expression of ZO-1, RPE65, and MerTK in post-confluent cultures of ARPE-19 cells. The cells were cultured for either one day or seven days. Whole-cell lysates were prepared and analyzed with immunoblotting using anti-ZO-1, anti-RPE65, anti-MerTK, and anti-GAPDH antibodies. (B) Quantification of protein expression levels of ZO-1, RPE65, and MerTK. The optical density of the Western blot bands obtained for ZO-1, RPE65, MerTK, and GAPDH were analyzed. The results are represented as the mean ± SEM. The differences in the protein level of ZO-1, RPE65, and MerTK between groups were compared using the paired t test. *** p < 0.001. (C) After one and seven days of culture, the characteristics of RPE cells, including tight junction proteins (ZO-1) and differentiation markers (RPE65) were identified by immunofluorescence staining. Magnification, ×400. Scale bar: 20 μm. Effects of glucosamine (GlcN) on phagocytosis of POS in ARPE-19 cells. (D) After seven days of cultures, ARPE-19 cells were pre-treated with or without GlcN (2.5, 5, 10, and 20 mM) for 24 h, followed by co-treatment with fluorescein isothiocyanate-labeled POS (FITC–POS) and the indicated concentration of GlcN (2.5, 5, 10, and 20 mM) for 3 h. The fluorescence intensity was measured using a microplate reader and normalized to the control group. The data are represented as mean ± SEM. ns, not significant; ** p < 0.01 versus POS group. (E,F) After seven days of culture, cells were pre-treated with or without GlcN (2.5, 5, 10, and 20 mM) for 24 h, and then co-treated with FITC–POS and the indicated concentration of GlcN (2.5, 5, and 10 mM) for: 3 h (E); and 24 h (F). The mean fluorescence intensity was measured by flow cytometry and normalized to control cells. The data are represented as mean ± SEM; ns, not significant versus POS group.