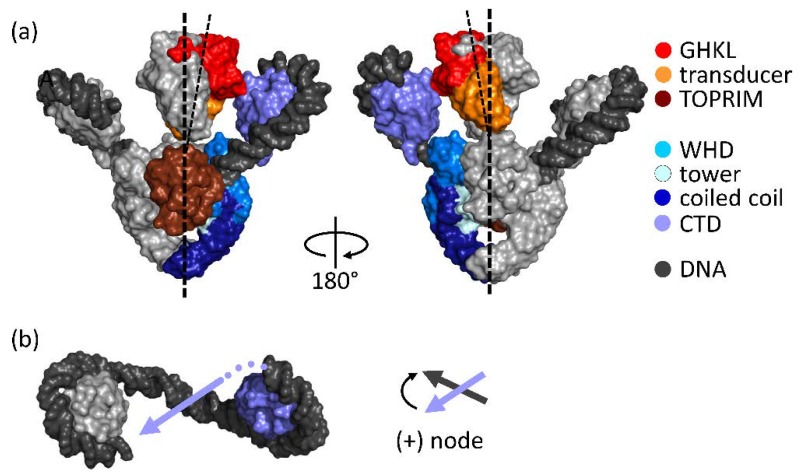
Figure 2.
Domain organization and structure of gyrase bound to DNA. (a) Cryo-EM structure of Thermus thermophilus gyrase in complex with ADPNP, DNA, and ciprofloxacin [20] (surface representation, left: front view, right: back view). One GyrA and one GyrB subunit are depicted in a color code representing the individual domains, the second GyrA and GyrB subunits are shown in gray. GyrA consists of an N-terminal domain (NTD) and a C-terminal domain (CTD, violet). The NTD comprises the winged-helix domain (WHD, light blue) with the catalytic tyrosine, the tower domain (cyan), and the coiled coil domain (dark blue). GyrB consists of an ATPase domain of the GyrB-Hsp90-histidine/serine protein kinases-MutL (GHKL, red) family, a transducer domain (orange), and a topoisomerase-primase (TOPRIM) domain (dark red). The DNA is depicted in dark gray. The two CTDs of the GyrA subunits are in different positions with respect to the NTD dimer. The GyrB subunits, locked in the dimeric, nucleotide-bound state, are tilted away from the two-fold axis of the NTD dimer (broken lines), and are inclined towards the CTD that is positioned to guide the T-segment into the upper cavity between the GyrB arms (see also (b)); (b) Top view of the two CTDs and the DNA (dark gray) wrapped in a positive supercoil. The right-hand side CTD (violet) is positioned such that the exiting DNA (violet arrow) could serve as a T-segment. The T- and G-segment then form a positive (+) node. Dark gray arrow: G-segment.