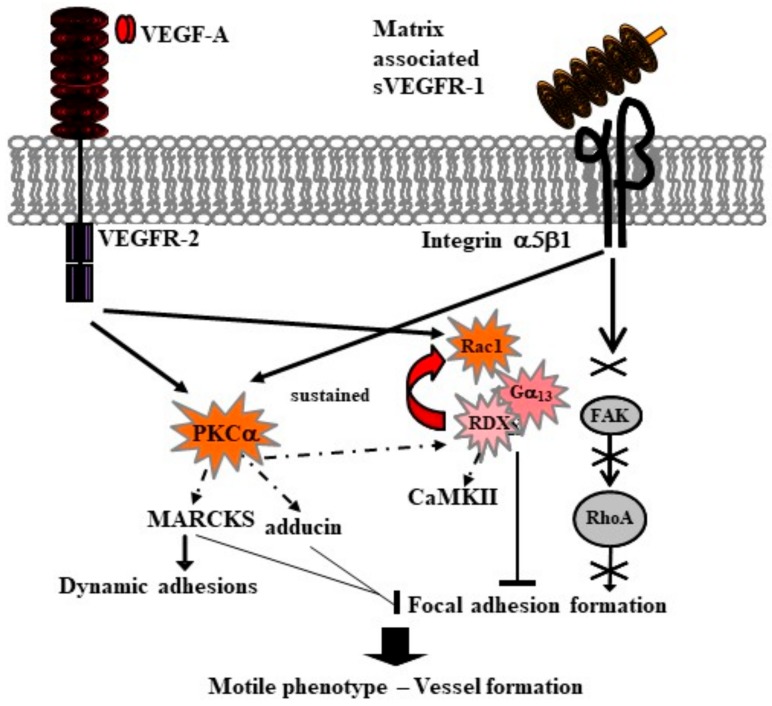
Figure 3.
Molecular signals of motility are activated by endothelial cell adhesion to matrix sVEGFR-1 through α5β1 integrin. Protein kinase C alpha (PKCα) phosphorylation by VEGFR-2 and/or α5β1 integrin (arrows) modulates both expression and phosphorylation (dotted arrow) of its substrates adducin, myristoylated alanine-rich protein kinase C substrate (MARCKS), and radixin (RDX) (see text). VEGFR-2 starts a signaling loop that involves the Rho family GTPase Rac1 and the heterotrimeric G protein α13 (Gα13), and is sustained (red arrow) by the activation of radixin, which also phosphorylates the calcium/calmodulin-dependent protein kinase II (CaMKII). Cell adhesion to matrix sVEGFR-1 does not activate the focal adhesion kinase (FAK) signaling (cross and crossed arrows). As a consequence, focal adhesion formation is blocked (bars). MARCKS expression is increased, and non-phosphorylated MARCKS accumulates into and stabilizes dynamic adhesions (black arrow). Thus, endothelial cells acquire a highly motile phenotype that allows them to initiate new vessel formation (thick black arrow).