Fig 6. Alterations in ergosterol pools and dynamics at the PM in Δ-s-tether cells.
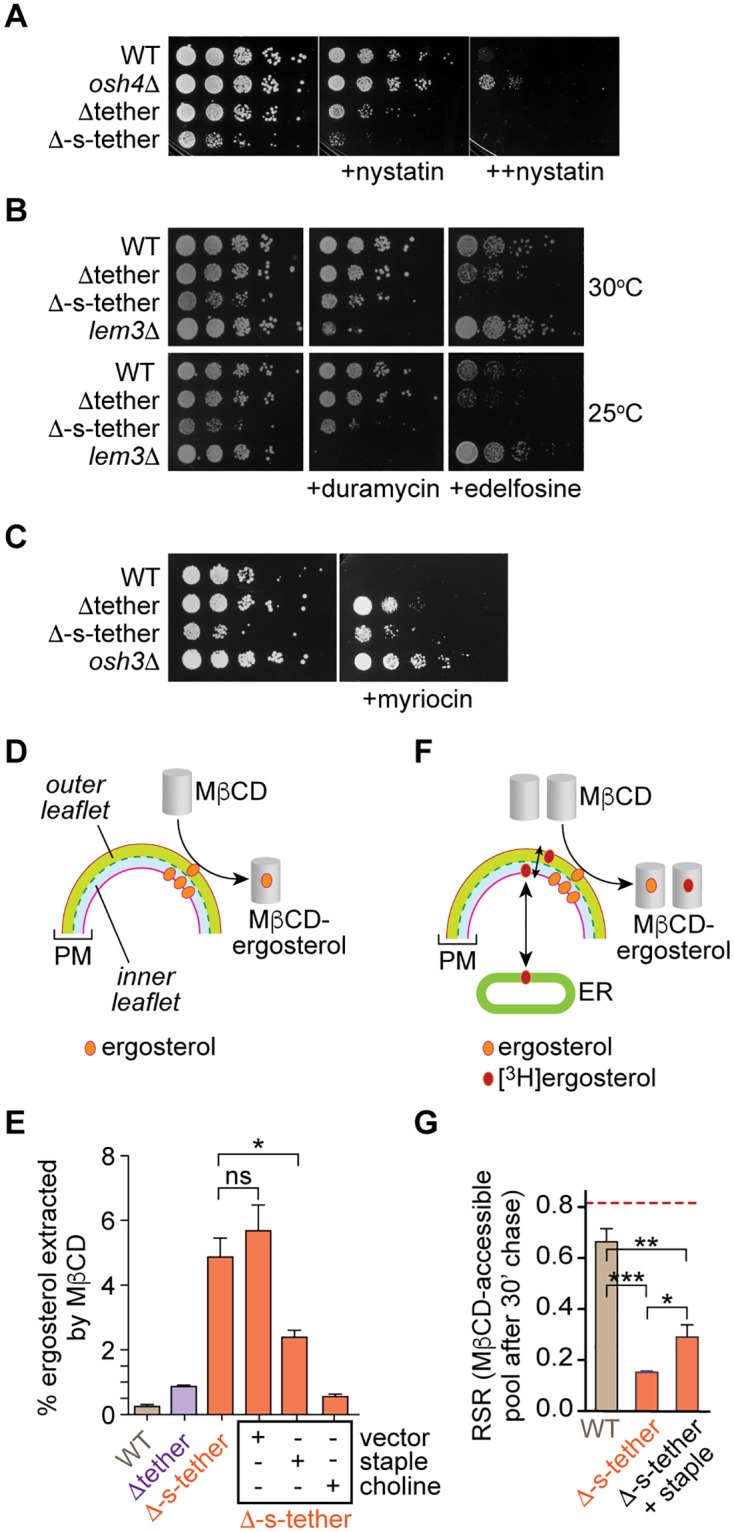
A. Sensitivity of Δ-s-tether cells to nystatin. Tenfold serial dilutions of WT (SEY6210), osh4Δ (HAB821), Δtether (ANDY198), and Δ-s-tether (CBY5838) cultures spotted onto solid rich medium containing no nystatin, 1.25 μM (+) nystatin, or 2.5 μM (++) nystatin and incubated for 3 d at 30 °C. B. Tenfold serial dilutions of WT, Δtether, and Δ-s-tether, lem3Δ (CBY5194) cultures were spotted onto solid rich media containing no drug, 5 μM duramycin, or 60 μM edelfosine and incubated for 2 d at 25 °C and 30 °C. The lem3Δ strain is known to be duramycin-sensitive and was used as a positive control. C. Tenfold serial dilutions of WT, Δtether, Δ-s-tether, and osh3Δ (JRY6202) cultures were spotted onto solid rich media containing no drug or 0.5 μg/mL myriocin and incubated for 2 d at 30 °C. The osh3Δ strain is known to be myriocin resistant and was used as a positive control. D. Assay to measure the proportion of cellular ergosterol that is extracted by MβCD. The PM of a yeast cell is shown, with outer (green) and inner (blue) leaflets delineated. Incubation of cells with MβCD on ice results in extraction of ergosterol from the outer leaflet. The sample is centrifuged to recover MβCD-ergosterol complexes in the supernatant. Ergosterol is extracted from the cell pellet and supernatant with hexane/isopropanol and quantified by HPLC (UV detection). E. The MβCD-accessible pool of ergosterol (quantified as in panel D) is about 20-fold greater in Δ-s-tether cells versus WT cells, and partially restored to WT levels in cells expressing the “ER-PM staple.” The statistical significance of the difference between the measurement of WT cells and each of the different Δ-s-tether samples is p < 0.0001, and between the Δ-s-tether samples is p = 0.0205 (*) and 0.436 (ns). F. Assay to measure transport of newly synthesized ergosterol from the ER to the MβCD-accessible pool. Cells are pulse-labeled with [3H]methyl-methionine to generate [3H]ergosterol in the ER, and chased as described in Fig 3. After a 30 min chase period, energy poisons are added and cells are placed on ice and incubated with MβCD. The ratio of the specific radioactivity of ergosterol in MβCD-ergosterol complexes versus that of the cell homogenate (RSR) provides a measure of transport. G. Transport of newly synthesized ergosterol from the ER to the MβCD-accessible pool. The bar chart shows RSR values for the different samples. The dotted line indicates the average RSR (about 0.82, averaged over both WT and Δ-s-tether samples) after 30 min of chase for the PM fraction, as described in Fig 3. The statistical significance was determined by one-way ANOVA (***p = 0.0003, **p = 0.0027, *p = 0.043). Numerical data presented in this figure may be found in S1 Data. Δ-s-tether, Δ-super-tether; ER, endoplasmic reticulum; HPLC, high-performance liquid chromatography; MβCD, methyl-β-cyclodextrin; ns, not significant; PM, plasma membrane; RSR, relative specific radioactivity; UV, ultraviolet; WT, wild type.
