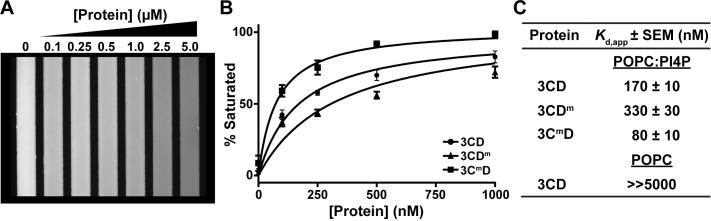
Fig 8. 3CD derivatives exhibit modulated binding to PI4P-containing membranes.
A microfluidics-based, membrane-binding assay was used to measure membrane binding by 3CD and derivatives thereof [47]. A bilayer is formed in the channels of the microfluidics device. The bilayer contains a phosphatidylcholine (POPC) and a fluorescent derivative of phosphatidylethanolamine (0.5 mol%) in the presence or absence of PI4P (7.5 mol%). Binding of the protein to the membrane quenches fluorescence, which enables measurement of the dissociation constant, Kd,app. (A) Image of the fluorescence observed in the channels of the device as a function of 3CD concentration using a 10X objective. (B) The change in fluorescence was converted to percent of bilayer saturated (see Materials and Methods) and plotted as a function of protein concentration. Data were fit to a Langmuir binding isotherm with an n = 1 to obtain values for Kd,app. Three independent experiments were performed. Error bars represent standard error of the mean. (C) Values for Kd,app measured for 3CD and its derivatives on the indicated bilayer. When using a bilayer consisting of POPC and the fluorescence probe, no change in fluorescence was observed at a concentration as high as 5000 nM.