CONSPECTUS
One of the fundamental questions guiding research in the biological sciences is how cellular systems process complex physical and environmental cues and communicate with each other across multiple length scales. Importantly, aberrant signal processing in these systems can lead to diseases that can have devastating impacts on human lives. Biophysical studies in the past several decades have demonstrated that cells can respond to not only biochemical cues but also mechanical and electrical ones. Thus, the development of new materials that can both sense and modulate all of these pathways is necessary. Semiconducting nanostructures are an emerging class of discovery platforms and tools that can push the limits of our ability to modulate and sense biological behaviors for both fundamental research and clinical applications. These materials are of particular interest for interfacing with cellular systems due to their matched dimension with subcellular components (e.g., cytoskeletal filaments), and easily tunable properties in the electrical, optical and mechanical regimes. Rational design via traditional or new approaches, such as nanocasting and mesoscale chemical lithography, can allow us to control micro- and nanoscale features in nanowires to achieve new biointerfaces. Both processes endogenous to the target cell and properties of the material surface dictate the character of these interfaces.
In this Account, we focus on (1) approaches for the rational design of semiconducting nanowires that exhibit unique structures for biointerfaces, (2) recent fundamental discoveries that yield robust biointerfaces at the subcellular level, (3) intracellular electrical and mechanical sensing, and (4) modulation of cellular behaviors through material topography and remote physical stimuli. In the first section, we discuss new approaches for the synthetic control of micro- and nanoscale features of these materials. In the second section, we focus on achieving biointerfaces with these rationally designed materials either intra- or extracellularly. We last delve into the use of these materials in sensing mechanical forces and electrical signals in various cellular systems as well as in instructing cellular behaviors. Future research in this area may shift the paradigm in fundamental biophysical research and biomedical applications through (1) the design and synthesis of new semiconductor-based materials and devices that interact specifically with targeted cells, (2) the clarification of many developmental, physiological, and anatomical aspects of cellular communications, (3) an understanding of how signaling between cells regulates synaptic development (e.g., information like this would offer new insight into how the nervous system works and provide new targets for the treatment of neurological diseases), (4) and the creation of new cellular materials that have the potential to open up completely new areas of application, such as in hybrid information processing systems.
Graphical Abstract
INTRODUCTION
Semiconductor nanostructures1 are promising materials for use in both understanding fundamental biological processes and developing novel clinical therapeutics due to their tunable length scales and physical properties. Additionally, nanostructures are able to operate either in interconnected configurations or as freestanding objects, forming multiple functional biointerfaces.1–10 With appropriate compositions and structures, they can be both biocompatible and biodegradable.3,8,11,12 For instance, one can readily control the doping profiles and morphologies in silicon (Si) nanowires via a vapor–liquid–solid (VLS) growth process and specifically by modulating synthesis parameters such as pressure and temperature.6,13–20 These synthetic controls can effectively encode micro- and nanoscale features, yielding a large toolbox of Si-based biomaterials.21,22 Other semiconductor-based nanostructured materials or devices can also serve as both sensors and modulators of cellular behavior, including as probes for detecting cellular fluid dynamics23 and intracellular pressure,24 transducers for biofuel production,25,26 and delivery vehicles for nucleic acids.27,28
APPROACHES FOR THE RATIONAL DESIGN OF SEMICONDUCTOR NANOSTRUCTURES
Approaches for Synthetic Control of Micrometer or Sub-micrometer Scale Features and Functions
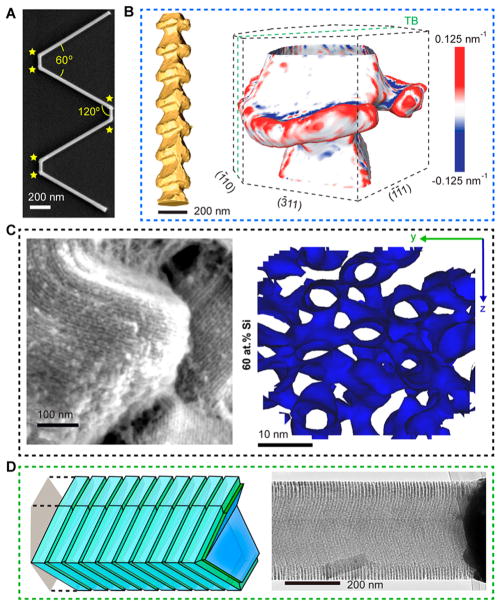
VLS growth of nanowires is an example of the type of precise control that can be achieved during nanomaterial synthesis. One study by Tian et al. demonstrated this concept by using pressure modulations in a chemical vapor deposition (CVD) system to synthesize 2D multiply kinked Si nanowires (SiNWs) (Figure 1A).6,14 Kinks confined to a single plane are introduced, at defined positions along SiNWs during the growth process via an abrupt modulation in growth pressure, yielding a nanostructure consisting of two straight single-crystalline segments oriented 60° apart,6,14 as shown in Figure 1A. This approach is unique in its ability to control the stereochemistry of adjacent kinks, paving the way for the synthesis of complex SiNW structures. This kinked morphology permits intracellular entry for electrical recordings of action potentials in excitable cells (vide infra).6,29
Figure 1.
New Si nanostructures can be explored for enhancing subcellular biointerfaces. (A) SEM image of a multiply kinked SiNW, showing six kinks (yellow stars) with individual angles of 120°. (B) STEM tomography of a Si spicule, showing anisotropic features (left); 3-D curvature map of one segment (right) shows convex and concave features in the spicule. “TB” denotes twin plane. Adapted with permission from ref 13. Copyright 2015 American Association for the Advancement of Science. (C) SEM (left) and 3D atom probe tomography isosurface (right) images of mesoporous Si particles, revealing periodic arrangements of Si nanowire assembly (left) and 3D structures interconnected by microbridges (right). The isosurface is plotted at 60 wt % Si. Adapted with permission from ref 8. Copyright 2016 Macmillan Publishers Ltd. (D) A schematic diagram (left) and a TEM image (right) of a SiNW with massively parallel sidewall grooves. Adapted with permission from ref 32. Copyright 2017 The Authors.
Another example of enabling angular features in nanowires uses the concept of metal diffusion along semiconductor surfaces that even in trace amounts can change the chemical etching behaviors of semiconductors.13 It has been demonstrated that during VLS growth of SiNWs, the gold (Au) catalyst can diffuse down the sidewalls of SiNWs30 under low silane partial pressure conditions. Luo et al. exploited this phenomenon by inducing periodic pressure modulations during SiNW growth to generate facet-selective and potassium hydroxide (KOH)-resistant bands of atomic Au on the surfaces of the SiNWs (Figure 1B).13 Both Au deposition and diffusion processes over SiNWs were found to be facet dependent, and thus Luo et al. were able to produce 3D graded Au/Si interfaces. KOH etching was then used to remove Si in regions unprotected by the atomic Au, creating Si spicules with skeleton-like morphologies, 3D tectonic motifs, and reduced symmetries (Figure 1B). These 3D nanowire sidewall features, similar to that of kinked nanowires, could potentially enhance biointegration subcellularly.
Approaches for Synthetic Control of Nanoscale Features
In addition to controlling micrometer and sub-micrometer scale features in single nanowires, synthetic control of secondary nanoscale features (the primary feature being the 1D nanowire geometry) is possible, allowing for fabrication of materials with unconventional geometries and unique structural properties. Jiang et al. employed a nanocasting approach to synthesize 3D nanoporous Si particles (Figure 1C).8 In this work, silane was decomposed using a CVD system inside the pores of an ordered mesoporous silica31 (SBA-15) template, consisting of hexagonally arranged channels. Wet chemical etching of the SBA-15 was used to generate unidirectionally aligned SiNW arrays interconnected by microbridges, as shown in Figure 1C. This material represents an example of a heterogeneous Si material with an amorphous atomic structure and ordered nanoscale framework. Approaches like this push the limits of conventional fabrication methods and allow for rational design of materials with novel capabilities.
Most recently, Fang et al. demonstrated that liquid Au–Si alloys established in classical VLS growth can deposit ordered 3D rings of isolated Au atoms over n-type phosphine-doped SiNW sidewalls.32 This ordered deposition results from dynamic spontaneous instability of the liquid alloy droplets, where droplet sidewall oscillations promote atomic Au deposition. They performed ab initio molecular dynamics simulations and unveiled, surprisingly, single atomic Au-catalyzed chemical etching of Si in a HF/H2O2 mixture. They subsequently verified this catalytic process in SiNWs and produced massive, ordered 3D grooves with spacings down to ~5 nm (Figure 1D). These nanoscale features can allow for unique optical and mechanical interfaces with cellular structures, in addition to serving as delivery or storage matrices. Additionally, these ordered nano-structures may provide uniform and controllable geometrical cues for transport and biophysical dynamics of subcellular components; however, this direction still requires many in-depth investigations.
Another important demonstration of rational material design with nanoscale features and functions was the realization of coaxial p-i-n SiNWs for photovoltaic applications.15,33 Here, introduction of dopant gases into VLS growth is used to create a nanoscale diode heterojunction in single SiNWs. p-i-n SiNWs, consisting of a p-type Si core with intrinsic and n-type Si shells, allow for charge separation to occur radially, yielding high efficiency carrier transport to the heterojunction, reducing bulk recombination.15,33 Further studies by Parameswaran et al. demonstrated the presence of atomic Au on the surface of these nanowires prepared with intentional Au diffusion.34 The atomic Au was shown to be integral to the production of photoelectrochemical currents from these SiNWs even in a free-standing configuration.
Open Questions
Can we use block copolymers as lithographic masks to fabricate nanowires with interesting meso- or nanoscale topographical features or heterogeneous surface chemical properties (alternating hydrophobicity/hydrophilicity35)?
What are the key topographical cues in Si nanostructures that promote intracellular targeting?
Are there new passivation or toxicity mitigation strategies for semiconductor nanowires, so that material compositions can be expanded far beyond Si, GaP, or InP?
ACHIEVING ROBUST BIOINTERFACES
Intracellular Biointerfaces
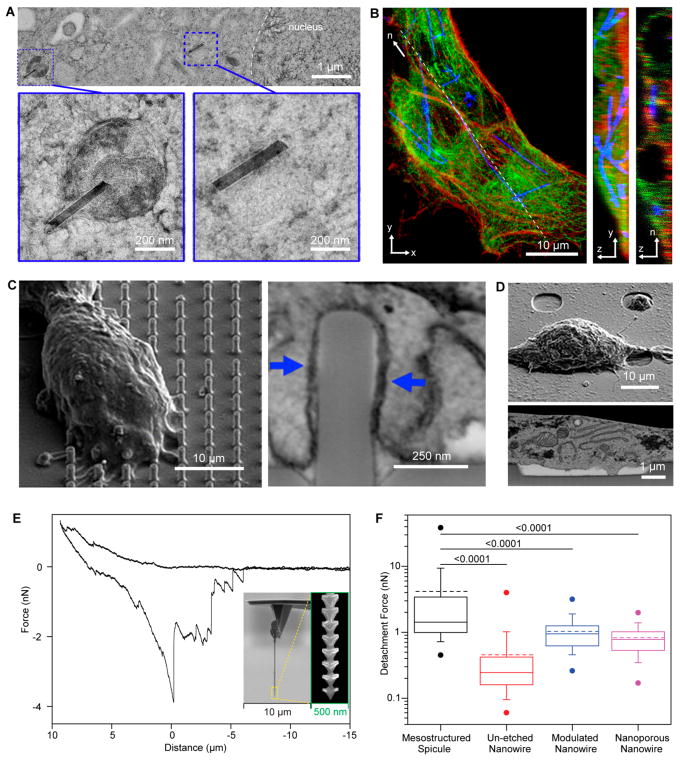
One method of achieving robust biointerfaces is making use of natural cellular processes, such as phagocytosis.3 Certain cell types, including macrophages and endothelial cells, are tasked with internalizing foreign materials and are thus able to uptake nanoscale materials such as SiNWs. Zimmerman et al. harnessed the phagocytic capabilities of human umbilical vein endothelial cells (HUVECs) to form robust interfaces between unlabeled SiNWs of varying diameters and HUVEC cells, as shown in Figure 2A,B. Subsequently, an active transport process allows for transport of nanowires to the peri-nuclear region inside the cell, where they cluster and are later packaged into lysosomes (Figure 2A).3 This work has paved the way for the future usage of unlabeled high-aspect-ratio semiconductors for intracellular modulation of cellular behavior.
Figure 2.
Nanostructured Si can enable tight biointerfaces in different cellular environments. (A,B) SiNWs can be internalized by mammalian cells. Adapted from ref 3. Copyright 2016 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-fnc/4.0/. (A) TEM image of HUVEC thin section (~250 nm thick), with higher magnification insets, illustrating the distribution of internalized wires, in both vesicles and the cytosol (scale bar 1 μm, inset scale bar 200 nm). (B) Confocal fluorescence micrograph of HUVECs (red, actin; green, tubulin) demonstrating SiNW internalization (blue, scattering). Maximum projection in the x–y plane (left, scale bar 10 μm), interpolated projection in the y–z plane (middle, height 3.5 μm), and thin confocal section taken along dashed line segment n (right, height 3.5 μm, length 48.3 μm). (C,D) Surface topography affects cleft distance between cell membrane and material surface. Adapted with permission from ref 40. Copyright 2017 American Chemical Society. SEM of plasticized HEK cells on nanopillar arrays (C) and nanopore arrays (D). (E,F) Si spicules can enhance biointegration at the tissue level. Adapted with permission from ref 13. Copyright 2015 American Association for the Advancement of Science. (E) Representative force–distance (F–D) curve collected by inserting and retracting an individual Si spicule mounted onto an AFM tip into and from a collagen matrix. Insets display the spicule-based AFM probe at different magnifications. (F) Box-and-whisker plots of forces required to detach Si spicules (black), unetched SiNWs (red), diameter-modulated SiNWs containing alternating segments of larger and smaller diameters axially down the wires as a result of alkaline etching (blue), and nanoporous SiNWs with pores 8.5 ± 4.4 nm in size resulting from Ag-assisted chemical etching (purple). Half of the data points are within the box and 80% are within the whiskers. Solid and dashed lines mark median and mean, respectively. Dots represent maximum and minimum values. The means of detachment force are 4.16 nN (mesostructured spicule), 0.455 nN (unetched nanowire), 1.03 nN (modulated nanowire), and 0.827 nN (nanoporous nanowire). N = 50, and numbers above bars indicate the P-value of the Mann–Whitney test.
Due to the inability of some cell types to phagocytose foreign materials, work has been done to still allow for the creation of intracellular biointerfaces for those cell types. Shi Kam et al. demonstrated that single-wall carbon nanotubes (SWNTs) functionalized with folate moieties could be selectively internalized into cancer cells expressing folate receptor tumor markers, allowing for targeted killing of cancer cells.36 Another study by Lee et al. demonstrated that SiNWs surface-functionalized with trans-activating transcriptional activator cell-penetrating peptides can be internalized into both primary hippocampal and dorsal root ganglion neurons.37 This study importantly demonstrates the ability of even nonphagocytic cells to form robust intracellular biointerfaces with nanoscale materials.
One challenge specific to intracellular biointerfaces, especially in complex tissue environments, is the ability to visualize individual nanostructures well. Adolfsson et al. provided a solution by using fluorescently barcoded GaP–GaInP axial nanowire heterostructures that could be identifiable in Drosophila fly gut tissue in a proof of concept experiment.38
Extracellular Biointerfaces
In addition to exploring intracellular biointerfaces, examining cell–material interfaces extracellularly is of interest especially if the target component of the cell is the plasma membrane and the interface must be quickly formed. Bianxiao Cui’s and Yi Cui’s Laboratories have demonstrated that nanopillar arrays can minimally invasively pin embryonic cortical neuronal cell bodies39 and, more recently, that plasma membranes of HL-1 cardiomyocytes cultured on a quartz substrate with nanopillars can deform to wrap around the pillars but will not readily deform outwardly around invaginating structures (Figure 2C,D).40 This differential membrane response to varying nanoscale topographical features must be considered during design of materials to be implanted into biological systems, as the tightness of the interface can vary significantly. At the membrane protein level, it was found that positively curved membranes are clathrin-mediated endocytosis (CME) hot spots. In particular, key CME proteins, such as clathrin and dynamin, are preferential for positively curved membranes,41 in contrast with many other membrane-associated proteins. Although these studies were performed primarily over quartz-based nanopillars, this concept can be applied to other semiconductor systems, suggesting that nanostructured semiconductor substrates can be used for investigating nanoscale topography-dependent processes in live cells.
Extracellular biointerfaces can also be enhanced by tuning mechanical properties of materials so that they match those of cellular components. Jiang et al. further explore this idea with their aforementioned 3D nanoporous Si particles.8 These particles, when immersed in aqueous environments, exhibit a mechanical stiffness that is similar to that of components of extracellular matrices (ECMs).8 The increased surface area and roughness of the nanoporous particles, as well as decreased mechanical stiffness in aqueous environments, allow these particles to achieve tight cellular interfaces with many mammalian cells.
Extracellular biointerfaces can also be tuned by external stimuli such as light. Pauzauskie et al. demonstrated a laser nanowire assembly method for locally manipulating and transporting nanowires to place one end of a 60 nm diameter cylindrical GaN nanowire against a HeLa cell membrane for several temporal durations.42 The ability to precisely arrange nanostructures with respect to their cellular targets can allow for meticulous control of biointerfaces.
Tissue-Level Biointerfaces
Tissue-level material interfaces can also be optimized during material synthesis. For example, Choi et al. demonstrated that soft materials like MoS2–graphene heterostructures can form tight, conformal interfaces with retinal tissue in vivo.43 We can also select for materials that can form close contacts with the ECM. Luo et al. demonstrated this concept with their anisotropic spicule SiNWs, which, like a bee stinger, are harder to retract from collagen matrices.13 This phenomenon was tested via atomic force microscopy (AFM) experiments in which spicules were mounted onto AFM tips and were subsequently inserted into and retracted from collagen matrices (Figure 2E). The detachment force measured for these spicules and was found to be an order of magnitude higher than that of smooth nanowires (4.16 nN compared to 0.455 nN, respectively) (Figure 2F).
Biodegradability without material fracturing is another important aspect of generating robust tissue level interfaces. Kang et al. developed a completely bioresorbable Si nanomembrane based device for intracranial pressure sensing.44 The various components of the material include thin layers of SiO2 and a nanoporous Si membrane, which can degrade at rates of 8 nm/day and 23 nm/day, respectively, and can dissolve completely without fracture.44
Open Questions
Can we achieve precise control in directing nanostructures to specific subcellular compartments using surface functionalization or light for phagocytic and non-phagocytic cells?
Can we achieve environmentally adaptable biointerfaces, for example, tight and loose interfaces on-demand?
DETECTING CELLULAR BEHAVIORS
Electrical Signaling Probes
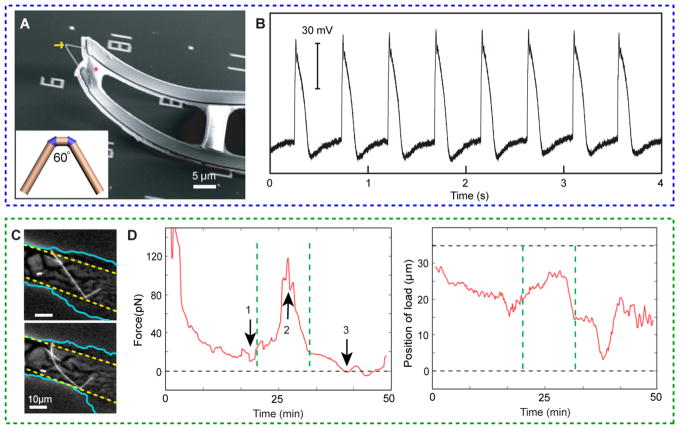
Once robust biointerfaces are achieved, materials can be used to detect cellular behaviors. These behaviors can include electrical and mechanical signaling in single cells or whole tissues. Electrical signaling probes in cellular systems are of great interest due to limitations in currently used technologies. While patch-clamp electrophysiology has revealed crucial mechanisms of biophysical behaviors in excitable cells, it is mechanically invasive and is limited in its capability to measure electrical signals for long time spans. Field effect transistors (FETs) are an attractive alternative to this technology as they can be fabricated at length scales more compatible with biological systems. Tian et al. took advantage of this and fabricated nanowire-FETs (NW-FETs) using the aforementioned kinked nanowires, consisting of two cis-linked kinked units with probe tip angles of 60°, with heavy n++ type doping for the source and drain arms (Figure 3A).6 These NW-FETs sense target biological species by coupling interactions with those species with the surface potential of the channel and thus the channel conductance. Here, kinked NW-FETs were used as 3D probes that could be inserted into cells (Figure 3A).6 These 3D probes are unique due to their nanoscale size, free-standing nature, and spatially separated FET and bulky interconnects, allowing for minimally invasive probing of electrical signaling in single cells. Electrical recordings, similar in quality to that of patch-clamp electrophysiology recordings, were demonstrated using these NW-FET probes in embryonic chicken cardiomyocytes (Figure 3B).6 Earlier work from Dr. Lieber’s lab oriented olfactory cortex slices over NW-FET arrays and produced extracellular recordings from various layers of cells.45 The ability to tune the spatial arrangement and density of the NW-FETs in the array allows for multiplexed measurements across many length scales, which is especially relevant to teasing out neural circuitry.45 These NW-FETs have now also been integrated into polymeric meshes and function well after being injected through a syringe into mouse brains.46
Figure 3.
SiNWs can be used for intracellular sensing. (A,B) Intracellular electrical recording with a field effect transistor. Adapted with permission from ref 6. Copyright 2010 American Association for the Advancement of Science. (A) SEM image of a flexible NW-FET-based recording device. Yellow arrow highlights a kinked nanowire, and the magenta star marks part of a polymer backbone used to support metal interconnects. Inset displays kinked NW-FET consisting of two cis-linked kinked units with a probe tip angle of 60°. (B) Representative trace of intracellular electrical recording from a beating cardiomyocyte. (C,D) Intracellular force sensing. (C) Optical micrographs of angiotensin II drug-induced HASMC contraction, showing straight (upper) and bent (lower) states (cyan, cell membranes; yellow, lamella boundary). Adapted with permission from ref 2. Copyright 2015 American Chemical Society. (D) Time-lapse force data (left) with coincident-load position (right), showing a well-defined contraction peak (between dashed green lines). Upon introduction of angiotensin II, a force minimum was observed due to relaxation of tension (arrow 1), followed by a contraction force peak (arrow 2), and a minimally strained state (arrow 3).
Mechanical Force Probes
Mechanical forces experienced by cells and tissues can play crucial roles in tissue and organ function, homeostasis, and development. The current most widely used techniques for probing these forces quantitatively both extracellularly and intracellularly include traction-force microscopy, optical tweezers, optical FRET sensors, and deformable material substrates.47–49 Many of these tools are invasive and have length scales that are incompatible with probing components of single cells. However, recent work has demonstrated that more precise, minimally invasive cellular force measurements can be achieved with nanoscale material probes.2,50,51 Prinz’s group demonstrated the use of GaP nanowire arrays to detect forces associated with neuronal growth cone lamelapodia dynamics down to 15 pN.51 Forces were measured by quantifying the displacement of fluorescently labeled nanowire tips caused by growth and movement of neurons cultured atop the array.
Recent work by Zimmerman et al. uses a completely free-standing method to show that kinked SiNWs can probe intracellular forces in both HUVECs and human aortic smooth muscle cells (HASMCs).2 Both of these cell types are phagocytic and, using endogenous pathways, can internalize singly kinked SiNWs.3 These singly kinked SiNWs were chosen so that the kink could be anchored to a part of the cytoskeleton, limiting rotational and translational movements of the nanowire (Figure 3C). Intracellular forces experienced by the cells were then measured during live-cell imaging of kinked SiNW bending that occurred as a result of both drug-induced contractions, as well as basal processes such as migration and division (Figure 3C,D). Forces were quantified using Euler–Bernoulli beam theory.
Open Questions
How can we achieve multiplexed sensing of intracellular signals in cellular circuits to truly map the signals generated by many cells types in communication with each other?
Besides electrical and mechanical signals, can semiconductor nanowires be used for intracellular temperature and chemical sensing from highly localized compartments?
INSTRUCTING CELLULAR BEHAVIOR
Topographical Cues to Instruct Behavior
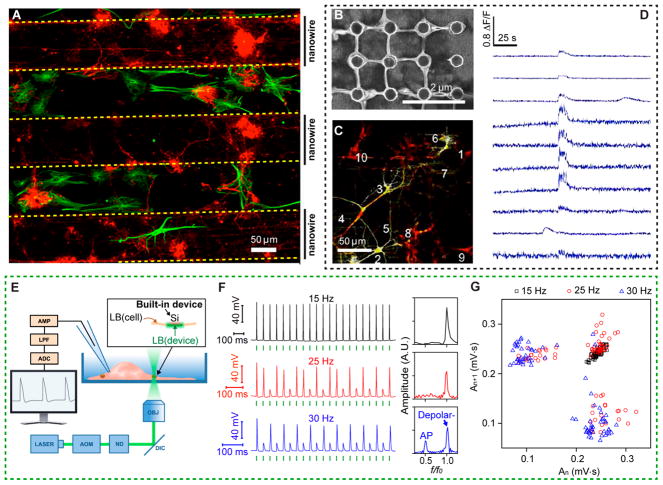
In addition to detecting cellular behavior, semiconductor materials interfacing with biological systems can instruct single or ensemble cell behavior. The naturally occurring cues that inform cellular growth, excitability, and communication can be chemical, mechanical, topographical, or even electrical in nature. Using biomaterials to mimic these naturally occurring cues can be crucial in treating diseases characterized by aberrant cues. Traditional methods for generating physical cues to instruct cellular behavior include artificial substrates that have been patterned with chemical components of ECM or surface topographies such as invaginations or pillars. Work by Prinz’s group showed that by fabricating substrates with patterned GaP nanowire regions, CNS neurons and glial cells can be spatially separated into distinct compartments (Figure 4A).4 The same group also showed that depending on the arrangement of the nanowires,4,5,52 retinal ganglion neuron processes could be aligned along nanowire rows, suggesting the possibility of using nanowires to promote contact guidance, a phenomenon by which substrate geometry can guide cellular outgrowth or movement of CNS neuronal processes. Daria’s group also developed an isotropic arrangement of high-aspect-ratio InP nanowires into scaffolds that can provide strictly physical cues for neurite growth and interconnected neuronal networks, as shown in Figure 4B.53 Neurite extension in hippocampal neurons can be guided by nanowire topography in these scaffolds, and as seen by calcium imaging studies, neuronal activity on the scaffold is highly coordinated (Figure 4C,D). These studies suggest new neural engineering methods through topography control.
Figure 4.
Si nanostructures can instruct cellular functions. (A) Neuronal cells (β-tubulin III, red) and glial cells (GFAP, green) on 100 μm wide bands with dense arrays of GaP nanowires (indicated by vertical lines and “nanowires”) separated by 100 μm wide bands with a flat topography after 18 DIV. Adapted with permission from ref 4. Copyright 2015 American Chemical Society. (B–D) Engineering highly interconnected neuronal networks on InP nanowire scaffolds. Adapted with permission from ref 53. Copyright 2017 American Chemical Society. (B) Neurite growth on an area of nanowires after 5 DIV showing anchoring and secondary branching of neurites at the nanowires. (C) Hippocampal neurons with correlated calcium signals. (D) ΔF/F vs time from numbered regions, suggesting high spatial correlation of neural activities as induced by the nanowire array. Top to bottom traces come from the regions of 1 to 10 in panel C. (E–G) Nanoporous Si particles for extracellular neuromodulation. Adapted with permission from ref 8. Copyright 2016 Macmillan Publishers Ltd. (E) Experimental setup used to elicit action potentials in DRG neurons by illuminating a single Si particle attached to a cell. Neurons were patch-clamped in the current-clamp, whole-cell mode. AOM, acousto-optic modulator; ND, neutral density filters; DIC, dichroic mirror; OBJ, microscope objective; AMP, amplifier; LPF, low-pass filter; ADC, analog-to-digital converter. Inset shows that a portion of the cell membrane functions as a built-in device. (F) Representative intracellular potential recordings of a DRG neuron to trains of laser pulses (5.32 μJ) at different frequencies, with corresponding FFTs (right). f and f 0 are output and input frequencies, respectively. Green bars indicate when laser pulses were delivered. (G) An area-based return map reveals an evolution of frequency-dependent 2D patterns. Data points are analyzed from 20 spikes per trial, 4 trials per frequency.
Electrical Cues to Instruct Behavior
The most widely used methods of modulating cellular electrical excitability include patch clamp electrophysiology and optogenetics.54,55 Semiconducting meso- and nanoscale materials are promising alternatives to these traditional methods given their minimally invasive and nongenetic nature. Jiang et al. used mesoporous Si particles to induce photothermal excitation of primary dorsal root ganglion neurons as shown in Figure 4E–G.8 After illuminating the plasma-membrane-supported Si particles, the fast photothermal effect from the Si induced a local temperature elevation, which subsequently caused a transient capacitance increase in the lipid bilayer and a depolarization of the bilayer due to capacitive current injection into the cells. Finally, recent work by Parameswaran et al. demonstrated photoelectrochemical modulation of primary dorsal root ganglion neuron activity with coaxial p-i-n SiNWs.34 This work represents an alternative optical method for modulating cellular excitability that does not produce large temperature increases.
Open Questions
Can we adopt existing processes used in other fields, such as energy and environmental sciences, to realize new pathways of biological modulation?
What would be the best strategy to combine semiconductor-based detection and modulation functionalities with internal feedback loops?
Can we modulate the bioelectric microenvironments for individual organelles and, to that end, probe the intrinsic bioelectric behaviors and their frequency-domain signatures?
OUTLOOK
Semiconductor nanostructures offer a promising direction for the synthesis of minimally invasive probes and manipulators of cellular behavior. Here, we have presented multiple approaches for the rational design of nanowire materials for the formation of robust biointerfaces with an ability to detect and instruct behaviors in cellular- and tissue-level systems. Some of the main challenges that remain include the design of materials that are mechanically compliant with native tissue, targeting of materials to specific subcellular organelles for intracellular studies, development of a platform that can sense and stimulate via a logic-gated feedback loop, the ability to perform multiplexing to investigate coordination between cells in complex circuits, and the ability to precisely control the lifetime and fouling of materials in physiological environments. Studies that continue to interface nanoscale materials with biological systems will deepen our understanding of how biological systems work and pave the way for novel life-saving therapeutics.
Biographies
Ramya Parameswaran grew up in Moraga, CA. She graduated from Stanford University with a B.S. with Honors in Chemical Engineering in 2010 and an M.S. in Chemical Engineering in 2011. Prior to joining the University of Chicago’s Medical Scientist Training Program (MSTP) in 2012, she worked in the Felsher Laboratory at Stanford and the Weiss Laboratory at UCSF. Her current work as a graduate student in Biophysical Sciences in the Tian and Adams Laboratories focuses on developing novel silicon-nanowire-based tools for the modulation of membrane voltage and the biophysical studies of signaling pathways in neurons, cardiomyocytes, and T cells. Ramya is a 2014 Paul and Daisy Soros Fellow as well as an NIH F30 awardee.
Bozhi Tian received his Ph.D. degree in physical chemistry from Harvard University in 2010. He is an assistant professor at the University of Chicago, working on semiconductor-enabled fundamental studies of subcellular biophysics and soft matter dynamics. Dr. Tian’s accolades from his independent career include the Inaugural ETH Materials Research Prize for Young Investigators (2017), Presidential Early Career Awards for Scientists and Engineers (2016), and TR35 honoree (2012).
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Zhang AQ, Lieber CM. Nano-Bioelectronics. Chem Rev. 2016;116:215–257. doi: 10.1021/acs.chemrev.5b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerman JF, Murray GF, Wang Y, Jumper JM, Austin JR, Tian B. Free-Standing Kinked Silicon Nanowires for Probing Inter- and Intracellular Force Dynamics. Nano Lett. 2015;15:5492–5498. doi: 10.1021/acs.nanolett.5b01963. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman JF, Parameswaran R, Murray G, Wang YC, Burke M, Tian BZ. Cellular uptake and dynamics of unlabeled freestanding silicon nanowires. Science Advances. 2016;2:e1601039. doi: 10.1126/sciadv.1601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piret G, Perez MT, Prinz CN. Support of Neuronal Growth Over Glial Growth and Guidance of Optic Nerve Axons by Vertical Nanowire Arrays. ACS Appl Mater Interfaces. 2015;7:18944–18948. doi: 10.1021/acsami.5b03798. [DOI] [PubMed] [Google Scholar]
- 5.Prinz CN. Interactions between semiconductor nanowires and living cells. J Phys : Condens Matter. 2015;27:233103. doi: 10.1088/0953-8984/27/23/233103. [DOI] [PubMed] [Google Scholar]
- 6.Tian BZ, Cohen-Karni T, Qing Q, Duan XJ, Xie P, Lieber CM. Three-Dimensional, Flexible Nanoscale Field-Effect Transistors as Localized Bioprobes. Science. 2010;329:830–834. doi: 10.1126/science.1192033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian BZ, Liu J, Dvir T, Jin LH, Tsui JH, Qing Q, Suo ZG, Langer R, Kohane DS, Lieber CM. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat Mater. 2012;11:986–994. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang YW, Carvalho-de-Souza JL, Wong RCS, Luo ZQ, Isheim D, Zuo XB, Nicholls AW, Jung IW, Yue JP, Liu DJ, Wang YC, De Andrade V, Xiao XH, Navrazhnykh L, Weiss DE, Wu XY, Seidman DN, Bezanilla F, Tian BZ. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nat Mater. 2016;15:1023–1030. doi: 10.1038/nmat4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu TM, Hong GS, Zhou T, Schuhmann TG, Viveros RD, Lieber CM. Stable long-term chronic brain mapping at the single-neuron level. Nat Methods. 2016;13:875. doi: 10.1038/nmeth.3969. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Fu TM, Cheng ZG, Hong GS, Zhou T, Jin LH, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo ZG, Fang Y, Lieber CM. Syringe-injectable electronics. Nat Nanotechnol. 2015;10:629. doi: 10.1038/nnano.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang SW, Park G, Edwards C, Corbin EA, Kang SK, Cheng HY, Song JK, Kim JH, Yu S, Ng J, Lee JE, Kim J, Yee C, Bhaduri B, Su Y, Omennetto FG, Huang YG, Bashir R, Goddard L, Popescu G, Lee KM, Rogers JA. Dissolution Chemistry and Biocompatibility of Single-Crystalline Silicon Nanomembranes and Associated Materials for Transient Electronics. ACS Nano. 2014;8:5843–5851. doi: 10.1021/nn500847g. [DOI] [PubMed] [Google Scholar]
- 12.Hwang SW, Tao H, Kim DH, Cheng HY, Song JK, Rill E, Brenckle MA, Panilaitis B, Won SM, Kim YS, Song YM, Yu KJ, Ameen A, Li R, Su YW, Yang MM, Kaplan DL, Zakin MR, Slepian MJ, Huang YG, Omenetto FG, Rogers JA. A Physically Transient Form of Silicon Electronics. Science. 2012;337:1640–1644. doi: 10.1126/science.1226325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo ZQ, Jiang YW, Myers BD, Isheim D, Wu JS, Zimmerman JF, Wang ZG, Li QQ, Wang YC, Chen XQ, Dravid VP, Seidman DN, Tian BZ. Atomic gold-enabled three-dimensional lithography for silicon mesostructures. Science. 2015;348:1451–1455. doi: 10.1126/science.1257278. [DOI] [PubMed] [Google Scholar]
- 14.Tian BZ, Xie P, Kempa TJ, Bell DC, Lieber CM. Single-crystalline kinked semiconductor nanowire superstructures. Nat Nanotechnol. 2009;4:824–829. doi: 10.1038/nnano.2009.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian BZ, Zheng XL, Kempa TJ, Fang Y, Yu NF, Yu GH, Huang JL, Lieber CM. Coaxial silicon nanowires as solar cells and nanoelectronic power sources. Nature. 2007;449:885–888. doi: 10.1038/nature06181. [DOI] [PubMed] [Google Scholar]
- 16.Yang C, Zhong ZH, Lieber CM. Encoding electronic properties by synthesis of axial modulation-doped silicon nanowires. Science. 2005;310:1304–1307. doi: 10.1126/science.1118798. [DOI] [PubMed] [Google Scholar]
- 17.Gudiksen MS, Lauhon LJ, Wang J, Smith DC, Lieber CM. Growth of nanowire superlattice structures for nanoscale photonics and electronics. Nature. 2002;415:617–620. doi: 10.1038/415617a. [DOI] [PubMed] [Google Scholar]
- 18.Lauhon LJ, Gudiksen MS, Wang CL, Lieber CM. Epitaxial core-shell and core-multishell nanowire heterostructures. Nature. 2002;420:57–61. doi: 10.1038/nature01141. [DOI] [PubMed] [Google Scholar]
- 19.Zhong ZH, Wang DL, Cui Y, Bockrath MW, Lieber CM. Nanowire crossbar arrays as address decoders for integrated nanosystems. Science. 2003;302:1377–1379. doi: 10.1126/science.1090899. [DOI] [PubMed] [Google Scholar]
- 20.Day RW, Mankin MN, Gao RX, No YS, Kim SK, Bell DC, Park HG, Lieber CM. Plateau-Rayleigh crystal growth of periodic shells on one-dimensional substrates. Nat Nanotechnol. 2015;10:345–352. doi: 10.1038/nnano.2015.23. [DOI] [PubMed] [Google Scholar]
- 21.Acaron Ledesma H, Tian BZ. Nanoscale silicon for subcellular biointerfaces. J Mater Chem B. 2017;5:4276–4289. doi: 10.1039/c7tb00151g. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerman J, Parameswaran R, Tian BZ. Nanoscale semiconductor devices as new biomaterials. Biomater Sci. 2014;2:619–626. doi: 10.1039/C3BM60280J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keren K, Yam PT, Kinkhabwala A, Mogilner A, Theriot JA. Intracellular fluid flow in rapidly moving cells. Nat Cell Biol. 2009;11:1219–1224. doi: 10.1038/ncb1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-Martinez R, Hernandez-Pinto AM, Duch M, Vazquez P, Zinoviev K, de la Rosa EJ, Esteve J, Suarez T, Plaza JA. Silicon chips detect intracellular pressure changes in living cells. Nat Nanotechnol. 2013;8:517–521. doi: 10.1038/nnano.2013.118. [DOI] [PubMed] [Google Scholar]
- 25.Kornienko N, Sakimoto KK, Herlihy DM, Nguyen SC, Alivisatos AP, Harris CB, Schwartzberg A, Yang PD. Spectroscopic elucidation of energy transfer in hybrid inorganic-biological organisms for solar-to-chemical production. Proc Natl Acad Sci U S A. 2016;113:11750–11755. doi: 10.1073/pnas.1610554113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakimoto KK, Wong AB, Yang PD. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science. 2016;351:74–77. doi: 10.1126/science.aad3317. [DOI] [PubMed] [Google Scholar]
- 27.Campagnolo P, Chiappini C, Leonardo V, Becce M, Perbellini F, Terracciano C, Smart N, Harding SA, Stevens MM. Porous silicon nanoneedles for localised in situ gene transfer for cardiac therapy. Cardiovasc Res. 2016;111:S87–S87. [Google Scholar]
- 28.Chiappini C, De Rosa E, Martinez JO, Liu X, Steele J, Stevens MM, Tasciotti E. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nat Mater. 2015;14:532–539. doi: 10.1038/nmat4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qing Q, Jiang Z, Xu L, Gao RX, Mai LQ, Lieber CM. Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions. Nat Nanotechnol. 2013;9:142–147. doi: 10.1038/nnano.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannon JB, Kodambaka S, Ross FM, Tromp RM. The influence of the surface migration of gold on the growth of silicon nanowires. Nature. 2006;440:69–71. doi: 10.1038/nature04574. [DOI] [PubMed] [Google Scholar]
- 31.Wan Y, Zhao DY. On the controllable soft-templating approach to mesoporous silicates. Chem Rev. 2007;107:2821–2860. doi: 10.1021/cr068020s. [DOI] [PubMed] [Google Scholar]
- 32.Fang Y, Jiang YW, Cherukara MJ, Shi FY, Koehler K, Freyermuth G, Isheim D, Narayanan B, Nicholls AW, Seidman DN, Sankaranarayanan SKRS, Tian BZ. Alloy-assisted deposition of three-dimensional arrays of atomic gold catalyst for crystal growth studies. Nat Commun. 2017;8:2014. doi: 10.1038/s41467-017-02025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kempa TJ, Cahoon JF, Kim SK, Day RW, Bell DC, Park HG, Lieber CM. Coaxial multishell nanowires with high-quality electronic interfaces and tunable optical cavities for ultrathin photovoltaics. Proc Natl Acad Sci U S A. 2012;109:1407–1412. doi: 10.1073/pnas.1120415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parameswaran R, Carvalho-de-Souza JL, Jiang YW, Burke M, Zimmerman J, Koehler K, Phillips A, Yi J, Adams E, Bezanilla F, Tian BZ. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat Nanotechnol. 2018;13:260. doi: 10.1038/s41565-017-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almquist BD, Melosh NA. Fusion of biomimetic stealth probes into lipid bilayer cores. Proc Natl Acad Sci U S A. 2010;107:5815–5820. doi: 10.1073/pnas.0909250107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Kam NW, O’Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JH, Zhang AQ, You SS, Lieber CM. Spontaneous Internalization of Cell Penetrating Peptide-Modified Nanowires into Primary Neurons. Nano Lett. 2016;16:1509–1513. doi: 10.1021/acs.nanolett.6b00020. [DOI] [PubMed] [Google Scholar]
- 38.Adolfsson K, Persson H, Wallentin J, Oredsson S, Samuelson L, Tegenfeldt JO, Borgstrom MT, Prinz C. Fluorescent Nanowire Heterostructures as a Versatile Tool for Biology Applications. Nano Lett. 2013;13:4728–4732. doi: 10.1021/nl4022754. [DOI] [PubMed] [Google Scholar]
- 39.Xie C, Hanson L, Xie WJ, Lin ZL, Cui BX, Cui Y. Noninvasive Neuron Pinning with Nanopillar Arrays. Nano Lett. 2010;10:4020–4024. doi: 10.1021/nl101950x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santoro F, Zhao WT, Joubert LM, Duan LT, Schnitker J, van de Burgt Y, Lou HY, Liu BF, Salleo A, Cui LF, Cui Y, Cui BX. Revealing the Cell-Material Interface with Nanometer Resolution by Focused Ion Beam/Scanning Electron Microscopy. ACS Nano. 2017;11:8320–8328. doi: 10.1021/acsnano.7b03494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao WT, Hanson L, Lou HY, Akamatsu M, Chowdary PD, Santoro F, Marks JR, Grassart A, Drubin DG, Cui Y, Cui BX. Nanoscale manipulation of membrane curvature for probing endocytosis in live cells. Nat Nanotechnol. 2017;12:750. doi: 10.1038/nnano.2017.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pauzauskie PJ, Radenovic, Trepagnier E, Shroff H, Yang P, Liphardt J. Optical trapping and integration of semiconductor nanowire assemblies in water. Nat Mater. 2006;5:97–101. doi: 10.1038/nmat1563. [DOI] [PubMed] [Google Scholar]
- 43.Choi C, Choi MK, Liu S, Kim MS, Park OK, Im C, Kim J, Qin X, Lee GJ, Cho KW, Kim M, Joh E, Lee J, Son D, Kwon SH, Jeon NL, Song YM, Lu N, Kim DH. Human eye-inspired soft optoelectronic device using high-density MoS2-graphene curved image sensor array. Nat Commun. 2017;8:1664. doi: 10.1038/s41467-017-01824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang SK, Murphy RKJ, Hwang SW, Lee SM, Harburg DV, Krueger NA, Shin J, Gamble P, Cheng H, Yu S, Liu Z, McCall JG, Stephen M, Ying H, Kim J, Park G, Webb RC, Lee CH, Chung S, Wie DS, Gujar AD, Vemulapalli B, Kim AH, Lee KM, Cheng J, Huang Y, Lee SH, Braun PV, Ray WZ, Rogers J. Bioresorbable silicon electronic sensors for the brain. Nature. 2016;530:71–76. doi: 10.1038/nature16492. [DOI] [PubMed] [Google Scholar]
- 45.Qing Q, Pal SK, Tian B, Duan X, Timko BP, Cohen-Karni T, Murthy VN, Lieber CM. Nanowire transistor arrays for mapping neural circuits in acute brain slices. Proc Natl Acad Sci U S A. 2010;107:1882–1887. doi: 10.1073/pnas.0914737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Fu TM, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo Z, Fang Y, Lieber CM. Syringe-injectable electronics. Nat Nanotechnol. 2015;10:629–636. doi: 10.1038/nnano.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bippes CA, Muller DJ. High-resolution atomic force microscopy and spectroscopy of native membrane proteins. Rep Prog Phys. 2011;74:086601. [Google Scholar]
- 48.Zheng XYR, Zhang X. Microsystems for cellular force measurement: a review. J Micromech Microeng. 2011;21:054003. [Google Scholar]
- 49.Addae-Mensah KA, Wikswo JP. Measurement techniques for cellular biomechanics in vitro. Exp Biol Med. 2008;233:792–809. doi: 10.3181/0710-MR-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanson L, Zhao WT, Lou HY, Lin ZC, Lee SW, Chowdary P, Cui Y, Cui BX. Vertical nanopillars for in situ probing of nuclear mechanics in adherent cells. Nat Nanotechnol. 2015;10:554–562. doi: 10.1038/nnano.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hallstrom W, Lexholm M, Suyatin DB, Hammarin G, Hessman D, Samuelson L, Montelius L, Kanje M, Prinz CN. Fifteen-Piconewton Force Detection from Neural Growth Cones Using Nanowire Arrays. Nano Lett. 2010;10:782–787. doi: 10.1021/nl902675h. [DOI] [PubMed] [Google Scholar]
- 52.Persson H, Li Z, Tegenfeldt JO, Oredsson S, Prinz CN. From immobilized cells to motile cells on a bed-of-nails: effects of vertical nanowire array density on cell behaviour. Sci Rep. 2016;5:18535. doi: 10.1038/srep18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gautam V, Naureen S, Shahid N, Gao Q, Wang Y, Nisbet D, Jagadish C, Daria VR. Engineering Highly Interconnected Neuronal Networks on Nanowire Scaffolds. Nano Lett. 2017;17:3369–3375. doi: 10.1021/acs.nanolett.6b05288. [DOI] [PubMed] [Google Scholar]
- 54.Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci. 2017;18:222–235. doi: 10.1038/nrn.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nat Neurosci. 2013;16:805–815. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]