Fig. 1. Crystal structure of the HisLsrK/HPr/ATP complex.
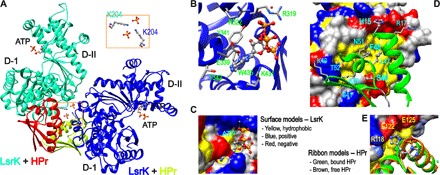
(A) The crystal asymmetric unit contained two molecules of the HisLsrK/HPr complex, in which the contact between two HisLsrK/HPr units is maintained by the ionic interaction mediated by two phosphate and LsrK-K204 residues (boxed region). (B) The ATP molecule seems to bind LsrK mainly via the hydrophobic interaction mediated by the adenine base, and the phosphate groups participate in additional hydrogen bonds and ionic interactions. The presence of hydrogen bonds is indicated with cyan lines, and the slightly longer distances than the limit of a hydrogen bond are indicated with orange lines. (C) The base of ATP fits into the hydrophobic pocket of LsrK. (D) The residues of HPr that participate in the interaction with LsrK are represented in the green ribbon model. F48 fits into the deep hydrophobic pocket of LsrK, and the neighboring L47 also participates in this hydrophobic interaction. HPr-H15 is located in the interface of the HisLsrK/HPr complex. (E) A structural overlay of free HPr and that in the HisLsrK/HPr complex suggests that H15 has structural flexibility, and the steric hindrance induced by the phosphorylated H15 may not be stringent. The phosphate group is simply attached to the H15-ND1 atom of the free HPr form for more clear comparison. The residues of LsrK that are located close to HPr-H15 are labeled yellow (R118, 4.15 Å; E122, 2.65 Å; E125, 4.50 Å).
