Fig. 3. Enzyme activity of LsrK is inhibited by HPr.
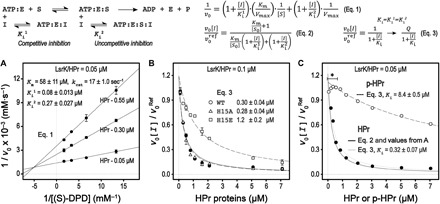
An enzymatic model of the LsrK and HPr interactions is summarized at the top. (A) Enzyme kinetic studies of LsrK were performed with a fixed ATP concentration (0.75 mM). The Lineweaver-Burk plots of LsrK activities in the presence of the HPr protein show that HPr inhibited LsrK activity via a combined manner of competitive and uncompetitive inhibition, as noted. Competitive inhibition (Ki1) was more substantive than uncompetitive inhibition (Ki2). (B) The relative activity of LsrK was measured as increasing the concentration of the wild-type (WT) and mutant HPr proteins. The concentrations of (S)-DPD and ATP were fixed at 3 and 1 mM, respectively. The reaction reference already contained the copurified HPr at a molar equivalent of LsrK (0.1 μM). Although the inhibition curves were not completely fitted by a simple inhibition equation (Eq. 3), inhibition activities of the HPrH15E and HPrH15A mutants were about five times less than and similar to that of the WT HPr, respectively. (C) The relative inhibition of LsrK activity by the native HPr and p-HPr proteins was assessed with 0.75 mM (S)-DPD and ATP. The relative inhibition of LsrK activity by the native HPr protein was described using the parameters determined in (A) (Eq. 2). The comparison of the relative inhibition activities between HPr and p-HPr was analyzed by using a simple inhibition equation (Eq. 3). The inhibition activity of p-HPr was much lower (more than 25 times) than that of the native HPr. The higher LsrK activity in the case of the p-HPr inhibition (marked with an asterisk) likely resulted from the phosphorylation of the copurified HPr with the LsrK protein by the EI enzyme that was retained in the p-HPr solution.
