Fig. 6. Structural comparison of HisLsrK with ecGK and ecXK.
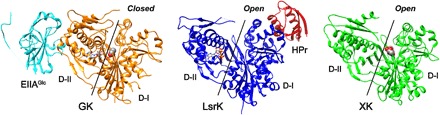
GK and XK are the members of the sugar kinases/Hsp70/actin superfamily and consist of two domains, in which the substrate sugar and ATP molecules bind to domain I (D-I) and domain II (D-II), respectively. ADP and ATP molecules in GK and HisLsrK are shown in the ball-and-stick model, and the glycerol and xylulose in GK and XK are represented as spheres. The structure of ecGK (PDB code, 1GLB) corresponds to the closed form, which allows the reaction of the phosphate transfer from the ATP to the glycerol. The native EIIAGlc binds to domain I and has been proposed to inhibit the enzyme activity through long-range conformational changes. On the other hand, the structures of HisLsrK and ecXK (PDB code, 2ITM) are open form, in which the distance between the ATP and the substrate molecules is too far for the enzyme reaction. HPr binds domain I of HisLsrK, instead of domain II.
