Abstract
Alcohol (ethanol) is one of the most widely used psychoactive substances worldwide. Alcohol consumption during pregnancy may result in a wide range of morphological and neurodevelopmental abnormalities termed fetal alcohol spectrum disorders (FASD), with the most severe cases diagnosed as fetal alcohol syndrome (FAS). FAS and FASD are not readily curable and currently represent the leading preventable causes of birth defect and neurodevelopmental delay in the US. The etiology of FAS/FASD remains poorly understood. This review focuses on the effects of prenatal alcohol exposure (PAE) on fetal cerebrovascular function. A brief introduction to the epidemiology of alcohol consumption and the developmental characteristics of fetal cerebral circulation is followed by several sections that discuss current evidence documenting alcohol-driven alterations of fetal cerebral blood flow, artery function, and microvessel networks. The material offers mechanistic insights at the vascular level itself into the pathophysiology of PAE.
Keywords: prenatal alcohol, fetal cerebral artery, maternal drinking, in utero alcohol exposure, fetal microvessel
Introduction
Normal brain development and function depend on a continuous blood supply. Complex cross-talk among neurons, glia, pericytes, and microvessels leads to the formation of a neurovascular functional unit and ensures that neuronal demands in nutritional supply and waste removal are met (Lecrux and Hamel, 2011). The local microvascular pressure is largely determined by cerebral arteries that are characterized by their ability to “autoregulate,” that is, to maintain basal vascular tone and blood pressure thus providing an adequate flow regardless of changes in systemic blood pressure (Faraci and Heistad, 1990; Paulson et al., 1990; Tan et al., 2013). Given the critical dependence of neuronal function on uninterrupted blood supply, it comes as no surprise that alterations of cerebral blood flow (CBF) lead to severe brain dysfunction. Indeed, the vascular contribution to prevalent neurological disorders has been increasingly recognized. For instance, vascular components have been described in cognitive impairment, dementia (Gorelick et al., 2011), Alzheimer’s disease (Humpel, 2011; Zlokovic, 2011), and even severe headaches (Ducros, 2012). In turn, loss of cerebrovascular autoregulation, e.g. during severe hypertensive encephalopathy, may result in catastrophic events, such as subarachnoid hemorrhage and hemorrhagic stroke (Heistad and Lawton, 1999).
Abnormalities in CBF (such as impaired autoregulation and fluctuating CBF velocity) have also been reported in high-risk neonates and preterm infants (Lou et al., 1979; Milligan, 1980; Perlman et al., 1983). Moreover, the immaturity of fetal cerebral regulation has been proposed as a prime factor in determining the severity of neurological deficits in preterm infants (Brew et al., 2014). Recent evidence points to the fetal cerebral circulation as a critical target of maternal drug use, including excessive alcohol consumption (Iida et al., 1994; Rao et al., 2007; Bake et al., 2012; Tobiasz et al., 2017). In this review, we will summarize the literature on the effects of prenatal (fetal) alcohol exposure (PAE) on fetal cerebral circulation. First, we will briefly recount the statistics and epidemiology of alcohol consumption. We will also present fetal alcohol syndrome (FAS) and fetal alcohol spectrum disorders (FASD) as lifelong consequences of PAE. Then, we will discuss the difficulties in studying the consequences of PAE, including its effect on the fetal cerebral circulation. A necessary overview of developmental and maturation milestones in fetal cerebral circulation will follow. Next, we will review current evidence that documents alcohol-driven alterations in fetal CBF, cerebral artery function, and microvessel networks. We will discuss possible mechanisms operating at the vascular level that drive the pathophysiology of PAE. The review will conclude with an outline of lingering questions that present current challenges and barriers in the field of PAE and fetal cerebral circulation.
Alcohol consumption
Alcohol (ethyl alcohol, ethanol) is one of the most widely consumed drugs worldwide (Ferreira and Willoughby, 2008). Apart from major psychoactive properties, alcohol consumption has profound effects on cardio- and cerebrovascular health; these effects have been reviewed elsewhere (Altura, 1984; Altura and Altura, 1984; O’Keefe et al., 2007; Reynolds et al., 2003). While amounts and patterns of alcohol consumption vary around the globe, binge drinking constitutes the most prevalent pattern of excessive alcohol consumption in the US (https://www.cdc.gov/alcohol/fact-sheets/binge-drinking.htm). Binge drinking is defined as a pattern of drinking that results in blood alcohol concentration (BAC) levels rising to 80 mg/dL (17.4 mM) (https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking). This typically occurs when 4 drinks are consumed by a woman or 5 drinks are consumed by a man in about 2 hours (https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking). It is estimated that one in six US adults binges on alcohol several times a month, with binge drinking being most prevalent among young adults of reproductive age between 18 and 34 years (Kanny et al., 2013). Thus, it comes as no surprise that alcohol is consumed by pregnant women. Indeed, studies in the US, Canada, Australia, and New Zealand report that 5 to over 50% of pregnant women engaged in alcohol drinking at some time during pregnancy (Flynn et al., 2003; Colvin et al., 2007; Ho and Jacquemard, 2009; Thanh and Jonsson, 2010). In addition, a recent analysis reveals that in selected geographic areas as much as 40–60% of pregnant women use some amount of alcohol (Lange et al., 2017; Popova et al., 2017).
Women who consume alcohol during pregnancy may engage in binge-drinking with BAC reaching 80 mg/dL (http://www.cdc.gov/media/releases/2015/p0924-pregnant-alcohol.html). Dependent on geographic area, the prevalence of binge-drinking pattern among pregnant women varies from a few digit percent to 50% (Lange et al., 2017; Popova et al., 2017). When women consume alcohol during pregnancy, maternal and fetal BAC time curves are nearly identical, with elimination of ethanol from the fetus being regulated primarily by the maternal clearance of ethanol (Brien et al., 1985; Hill et al., 1983). As documented by numerous studies, binge alcohol consumption with sharp and high peaks of BAC above 80 mg/dL produces the most negative effects on the developing fetus (Pierce and West, 1986; Maier and West, 2001; May and Gossage, 2011). As a result, PAE leads to a range of developmental abnormalities termed fetal alcohol spectrum disorders (FASD), with the most severe cases representing fetal alcohol syndrome (FAS) (Riley et al., 2011; Basavarajappa, 2015). The latter is characterized by intrauterine growth restriction, a characteristic craniofacial dysmorphism, and a set of behavioral and cognitive abnormalities (Archibald et al., 2001; Burd et al., 2010; Lewis et al., 2015; Carter et al., 2016). The prevalence of FAS/FASD varies across geographic regions. In countries with the highest percentage of alcohol consumption during pregnancy, FAS/FASD prevalence reaches 6%, with the global prevalence of FASD among children and youth being estimated at 0.77% (May and Gossage, 2001; May et al., 2009; Olivier et al., 2016; Lange et al., 2017; Popova et al., 2017). FASD is the leading preventable cause of neurodevelopmental disability in the US (Bailey and Sokol, 2008).
It is known that PAE targets multiple fetal organs and systems, including heart, kidney, liver, gastrointestinal tract, and the endocrine and immune systems (Kenna et al., 2012; Gauthier, 2015; Caputo et al., 2016). However, the brain constitutes the most severely affected organ, exhibiting both structural and functional abnormalities in response to PAE (Mattson et al., 2001; Caputo et al., 2016). The mechanisms leading to FAS/FASD remain poorly understood. Moreover, there is no readily available cure for the lifelong consequences of PAE (Murawski et al., 2015; Wilhoit et al., 2017).
Current obstacles in studying the consequences of PAE
The lack of mechanistic understanding of the FAS/FASD etiology stems in part from the difficulties related to the experimental design and experimental models of PAE. Studying the mechanisms underlying alcohol-induced dilation of fetal cerebral arteries is infeasible on humans for several reasons. First of all, intended exposure of human fetuses to alcohol is ethically unacceptable. Second, even with access to a maternal population, it is nearly impossible to standardize patterns and amounts of alcohol consumption during pregnancy. Much of the available information regarding alcohol consumption during pregnancy is obtained based on maternal self-reports (McCarthy et al., 2013; Smith et al., 2014; Lanting et al., 2015). This non-invasive approach has been found valid and reliable (Del Boca and Darkes, 2003; Lintonen et al., 2004). However, the accuracy may require further optimization due to the subjectivity, including under-reporting of drinking during pregnancy (Bakhireva and Savage, 2011; Manich et al., 2012). Third, there are many confounding factors in humans, including genetic background, maternal health history, diet, multi-substance abuse, and socioeconomic status (Patten et al., 2014).
Despite the limitations of human studies, they are indispensable for the identification and validation of biomarkers of PAE, such as ethyl sulfate in maternal urine, and fatty acid ethyl esters in neonatal hair and meconium (Chan et al., 2004; Bakhireva and Savage, 2011). Several other biomarkers of PAE, such as ethyl glucuronide (Gutierrez et al., 2015) and phosphatidylethanol (Bakhireva et al., 2014) have been put forth, yet there is still no selective and definitive marker for an early diagnosis of FAS/FASD (Joya et al., 2012; Bager et al., 2017).
Considering the limitations of human-based studies, PAE of laboratory animals have been widely used as an alternative. Thus, standardized alcohol exposure during timed pregnancy has been extensively utilized in several animal species including invertebrates, fish, rodents, sheep, and non-human primates, as extensively reviewed elsewhere (Wilson and Cudd, 2011; Patten et al., 2014; Murawski et al., 2015; Wang and Kroenke, 2015). These models, however, have limitations, including differences from humans in genetic background, alcohol metabolism, and the general characteristics of pregnancy itself. For example, the timing of neurodevelopmental milestones places the third trimester of human pregnancy equivalent to the early postnatal period in rodent species (Semple et al., 2013). In addition, the small size of rodents presents a major obstacle to harvest cerebral artery tissue for biochemical analysis and functional studies in vitro that could render mechanistic insights. Larger animals such as rabbits and pigs are suitable for harvesting fetal arteries; however, these species carry multiple fetuses making it difficult to obtain ultrasound evaluation of cerebral blood flow when several evaluations have to be done on each fetus (such as pre-ethanol and post-ethanol exposure). Ovine species, on the other hand, offer the research benefit of the large size of their fetuses and the additional advantage of singleton or twin pregnancies. In this model, BAC and, conceivably, the amount of alcohol reaching the cerebral circulation, can be easily determined. However, ruminal metabolism of ethanol is unique (Raun and Kristensen, 2011), and does not readily model the human in studies requiring gastric ingestion of alcohol. Finally, non-human primates are the closest to humans from an evolutionary perspective and cerebral development milestones (Stewart and Disotell, 1998; Pillay and Manger, 2007). Although non-human primates are widely utilized for research on alcohol consumption (Grant and Bennett, 2003; Jimenez and Grant, 2017), scarce availability of primates for research and ethical considerations limit the use of these species in invasive studies.
Fetal cerebrovascular development
Development of the cerebral circulation in humans starts with the formation of six pairs of branchial arch arteries, some of which give rise to the internal carotid artery, at approximately 24 days of gestation (Menshawi et al., 2015). Several days later, the internal carotid artery branches into anterior and posterior divisions that later give rise to basilar, anterior, middle, and posterior cerebral arteries (Menshawi et al., 2015). Blood vessels finally start to differentiate into arteries and veins at 10 weeks of gestation (Solonskii et al., 2008).
Several weeks later, the development of penetrating vessels starts from the meningeal plexus that already possesses a functional blood-brain barrier (Hagan and Ben-Zvi, 2015). The latter is formed very early in embryogenesis and protects fetal brain from unwanted blood-borne materials, supports the unique metabolic needs of the neuronal cells (neuroblasts and mature neurons), and plays a crucial role in maintaining brain homeostasis (Obermeier et al., 2013; Hagan and Ben-Zvi, 2015).
Several stages have been described in human cerebral microvessel network development. At 15 weeks, radially oriented branch-lacking arterial precursors originating from the leptomeningeal arteries can be detected through cortical matter up to the subcortical regions (Allsopp and Gamble, 1979; Norman and O’Kusky, 1986). At this time, nerve fibers containing noradrenaline and acetylcholinesterase have just appeared alongside basilar arteries (Kawamura and Takebayashi, 1994). By 20 weeks of gestation, horizontal arterial branches start penetrating the ventral cortex; some of them are ascending into the more superficial cortical layers. At 20–27 weeks, branching becomes apparent in the upper half of the cortex. Finally, from 27 weeks of gestation to term, small radial vessels appear in the most superficial cortical layer. While arterial branching within the cortical matter is developing, the number of radially oriented vessels on the pial surface of the brain declines. The development of the fine vessel (microvessel) networkis finalized after birth (Norman and O’Kusky, 1986).
At subcellular and molecular levels of analysis, cerebrovascular development represents a complex, multi-step process with a multitude of players involved. Their explicit consideration exceeds the scope and limits of this review, but is covered well elsewhere (Scher, 2012; Santhosh and Huang, 2015). In general terms, the expansion of the vascular network in the brain is largely achieved by angiogenesis, that is, the sprouting of new blood vessels from existing ones via cell migration (Carmeliet and Collen, 1998). Thus, vascularization requires effective proteolytic clearance of the surrounding tissue, loosening of existing inter-cellular contacts, and removing contact and support from other cell types to enable endothelial cell migration. In addition, migrating cells need to protrude extensions, change shape, and migrate to a new place according to guiding cues. The migration of smooth muscle progenitors and their differentiation must take place as well. Fusion of newly formed vessels with the existing network may also be needed. All in all, angiogenesis is orchestrated by a wide variety of structural and signaling molecules from different families. They include, but are not limited to, vascular endothelial growth factor (VEGF), integrins, fibronectin, vascular cell adhesion molecules, angiopoietins, tyrosine kinases TIE1 and TIE2/TEK, fibroblast growth factors, extracellular matrix proteins and proteinases, Wnt, and transforming growth factor-β signaling (Carmeliet and Collen, 1998; Ulrich et al., 2016; Wang et al., 2018). Vascular development also requires proper stop signals that may act by mere inhibition of angiogenesis-stimulating factors or by induction of vascular regressions, such as via tumor necrosis factor (TNF) signaling and apoptosis (Carmeliet and Collen, 1998).
From a physiological standpoint, the fetal cerebrovascular system differs in its function and responses to environmental stimuli from its adult counterpart. For example, fetal cerebrovascular arterioles possess high responsiveness to sympathetic stimulation, higher expression of the proliferating cell nuclear antigen, and higher density of L-type Ca2+-channels, a different pattern and density of noradrenaline and calcitonin-gene-related-peptide innervation, and different responses to hypoxia when compared to those from term fetuses or adults (Tsai et al., 1989; Wagerle et al., 1990; Blood et al., 2002; Zhao et al., 2004; Pearce, 2006). Adrenaline- and KCl-driven constriction of cerebral arteries is progressively increased from the second trimester equivalent to term, with concomitant increases in structural parameters (such as lumen diameter, media thickness, and media cross-sectional area) being detected (Goyal et al., 2012).
The unique features of the fetal cerebrovascular system merit a systematic investigation to detect relevant targets of PAE. While the toxic effects of alcohol on developing neurons and glia are relatively well studied (Drew and Kane, 2014; Basavarajappa, 2015), the recognition of the fetal cerebrovascular system as a critical target of alcohol is only emerging. It is significant that cerebrovascular development is synchronized with central neurogenesis (Carmeliet and Tessier-Lavigne, 2005). Such coordination is achieved by neurogenic factors that affect development of the vascular system and, conversely, vascular factors that contribute to neuronal development and plasticity (Scher, 2012). The former include ephrin-B2 and corresponding receptor EphB4 signaling, neurophilin receptor activators, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and microRNA-9 (Palmer and Klein, 2003; Scher, 2012; Madelaine et al., 2017). The latter are presented by VEGF, insulin-like growth factor (IGF-1), angiopoietin-I, fibroblast growth factor (FGF) (Virgintino et al., 2003; Carmeliet and Tessier-Lavigne, 2005; Scher, 2012). Thus, along with molecular targets that are unique to cerebrovascular development, PAE may alter signaling entities that coordinate vascularization and neurodevelopment.
Ethanol-driven alterations in fetal cerebrovascular function: transient effects
Ethanol-driven alterations in fetal cerebrovascular function are specific to this vascular bed and differ from the consequences of PAE on resistive vessels from other circulations (Parkington et al., 2014). This specificity is not surprising as alcohol action on adult vessels varies with the vascular network as well (Tabrizchi and Pang, 1993). The majority of reports document an increase in fetal cerebral perfusion and, conceivably, a drop in fetal cerebral artery blood flow Doppler velocity indices, during maternal alcohol consumption in vivo (Mann et al., 1975; Parnell et al., 2007; Kochunov et al., 2010; Bake et al., 2012; Seleverstov et al., 2017).
Kochunov et al. (2010) assessed the effect of a single episode of maternal alcohol intoxication on fetal brain blood perfusion using dynamic susceptibility contrast (DSC) magnetic resonance imaging on baboons at 24 weeks of gestation (168 days, term at 175). Alcohol at 3 g/kg was administered to pregnant baboons via gastro-nasal catheter, this administration resulted in a peak maternal BAC of 0.23% (over 200 mg/dL). Under these conditions, there was a four-fold increase in the peak contrast concentrations in the fetal brain when compared to the administration of contrast agent alone before alcohol administration. In addition, significant increases in the contrast uptake and washout rates in the fetal brain were detected (Kochunov et al., 2010). These findings were interpreted as ethanol evoking an increase in fetal CBF, which would be consistent with the alcohol-induced dilation of fetal cerebral vessels. An alternative explanation would be that alcohol induces an increase in fetal cerebral artery permeability to the contrasting agent gadodiamide used in the protocol (Kochunov et al., 2010). However, the former interpretation is consistent with the results obtained in our lab. In two independent studies, we used trace-free Doppler ultrasonography to detect changes in fetal cerebral artery function following baboon maternal alcohol intoxication with BAC reaching 80 mg/dL. This followed 1.5 mg/kg ethanol administration via oral gavage to pregnant baboon dams at 90, 100, and 110 days of gestation. This protocol resulted in a decreased peak systolic velocity of fetal anterior and middle cerebral arteries (Fig. 1) (Selevertsov et al., 2017; Tobiasz et al., 2017). A similar conclusion was reached in a murine model of pregnancy; Bake et al. (2012) have demonstrated changes in cranially directed blood flow of the developing fetus that persisted up to 24 hours following alcohol administration. In this study, pregnant mouse dams received 3 g/kg of ethanol on 12 days of gestation (second trimester equivalent of human pregnancy) either via oral gavage or via intraperitoneal injection (IP). These routes of ethanol administration resulted in maternal blood alcohol concentrations of 117 and 150 mg/dL, respectively. Regardless of the administration method, the authors observed a suppressive effect of ethanol on fetal vascular function. Both acceleration (in mm/sec2) and time-velocity integral (in mm3/sec) were significantly diminished by ethanol in fetal internal carotid and middle cerebral arteries. Alcohol-driven alterations in fetal CBF persisted for 24 hours following ethanol administration. In addition to quantitative suppression of fetal cranially directed blood flow, qualitative changes in the pattern of flow were observed in fetal middle and posterior cerebral arteries. The loss of time-velocity integral was supplemented by the loss of directional blood flow, as was evident by the turbulence of the flow. These changes occurred in the absence of any ethanol effect on fetal heart rate (Bake et al., 2012).
Figure 1. Drop in fetal cerebral blood flow velocity in presence of alcohol.
A. Original sonograms depicting Doppler waveforms from the middle cerebral artery of a fetal baboon. Sonograms on the top present waveforms before (Base, left) and 120 min after (right) the maternal infusion of control drink. Sonograms on the bottom present waveforms before (Base, left) and 120 min after (right) the maternal infusion of alcohol-containing drink. Infusions were performed on a pregnant baboon via gastric gavage as described (Seleverstov et al., 2017; Tobiasz et al., 2017). Maternal BAC reached on average 80 mg/dL and plateaued within 60 min from the infusion. B. Averaged data show statistically significant drop in fetal cerebral artery peak systolic velocity following maternal infusion of alcohol-containing drink when compared to the infusion of control drink (iso-caloric to alcohol).
In contrast to a multitude of reports describing alcohol-driven increases in fetal CBF, a decrease in CBF was reported following maternal infusion of 1 g/kg ethanol in near-term sheep (Richardson at al., 1985). The phenomenology of alcohol-driven alterations in fetal cerebrovascular function is becoming even more complicated, as ethanol effects on the cerebral blood flow were documented to be region-specific (Parnell et al., 2007). In this study, pregnant sheep at the third trimester equivalent were exposed to 0.75 or 1.75 g/kg ethanol to render maternal BAC of 85 or 185 mg/dL, respectively. The ethanol infusion was achieved by a catheter in the jugular vein. Control group received saline infusion. Both infusion paradigms lasted 3 days per week in succession starting from 109 until 132 days of gestation. The lower dose of alcohol did not render significant changes in fetal cerebral blood flow. This outcome is consistent with another report on pregnant sheep, documenting lack of changes in fetal CBF and cardiac parameters following 1 g/kg ethanol infusion into maternal blood via i.v. infusion at gestational day 92 (term at 145–150 days) with maternal BAC reaching 150 mg/dL (Gleason and Hotchkiss, 1992). In contrast, the higher dose (1.75 g/kg maternal weight) significantly increased CBF by over 30% compared to the infusion of control saline. In the cerebellum, increase in CBF 1 hour following the ethanol infusion was up to 50% higher than in controls (Parnell et al., 2007). The changes in fetal CBF were accompanied by changes in systemic hemodynamics: significant increase in fetal heart output and heart rate, and a reduction of mean arterial pressure and total peripheral resistance (Parnell et al., 2007). Summarizing the work on fetal in vivo cerebrovascular responses to PAE, there is a clear consensus that acute alcohol administration has an evident effect on cerebrovascular function. This effect is concentration-dependent (higher doses of ethanol evoke larger increases in CBF), with possibly region-specific susceptibility to ethanol action.
Notably, PAE not only evokes changes in CBF but also modifies CBF responses to environmental factors such as hypoxia (Mayock et al., 1985). In the study on pregnant sheep, maternal infusion of 1.5 g/kg ethanol during gestational days 60–90 (term at 145–150) rendered BAC of 214 mg/dL and led to attenuation of hypoxia-driven increase in CBF at gestational day 125 (Mayock et al., 1985). Thus, PAE alters adaptive changes in CBF to environmental insult. The role of the ethanol-driven increase in CBF in a PAE-associated developmental delay remains to be established. However, several pieces of indirect evidence suggest that such contributions could be profound. First, increases in CBF have been linked to substantial growth insufficiency. Indeed, an alcohol-unrelated pathology, spontaneous intracranial hypotension, is characterized by noticeable morphological abnormalities of the skull that result from an abnormal drop in cerebral artery tone (Gordon, 2009; Yoon et al., 2012). Craniofacial malformation is also a key diagnostic feature of FAS in humans (Jackson and Hussain, 1990), and can be replicated in animal models of FASD (Anthony et al., 2010). Second, alcohol-driven changes in fetal cerebral perfusion precede fetal growth restriction, as our lab demonstrated using Doppler ultrasonography on baboon fetuses following three episodes of maternal alcohol intoxication during second trimester equivalent (Tobiasz et al., 2017). Third, there is a positive association between severity of alcohol-driven alterations in CBF and degree of neuronal loss, as reported in an ovine model of pregnancy (Parnell et al., 2007): fetal alcohol intoxication during third trimester equivalent only increases CBF significantly in the cerebellum. Loss of central neurons was observed in cerebellum but neither in hippocampus nor the olfactory bulb, two regions that did not experience alterations in CBF (Parnell et al., 2007). Fourth, alterations in CBF are expected to compromise delivery of nutrients to and removal of metabolites from neurons. Thus, altered CBF is expected to result in modifications of neuronal development and function.
Alterations in the blood biochemical profile usually accompany and possibly could contribute to the pathological consequences of alcohol-driven disruption of CBF. Interestingly, reports describing the lack of alcohol effect on CBF (with low ethanol concentrations of 0.75 g/kg or 1 g/kg being studied) in an ovine model also attest to the lack of robust changes in fetal blood metabolites (Gleason and Hotchkiss, 1992; Parnell et al., 2007). Only mild hypoglycemia and a mild increase in lactate concentration in fetal blood were reported following 1 g/kg ethanol administration via maternal i.v. infusion at gestational day 92 (term at 145–150 days), with maternal BAC reaching 150 mg/dL (Gleason and Hotchkiss, 1992). These changes were accompanied by a mild increase in fetal blood oxygen saturation (Gleason and Hotchkiss, 1992). Another report using 1 g/kg ethanol in near-term sheep documented a decrease in VCO2, a measure of oxidative metabolism (Richardson et al., 1985). As ethanol concentration increased to 1.75 g/kg, an increase in the fetal arterial partial pressure of CO2 and a decrease in pH were documented in the ovine model following repeated alcohol administration during similar gestational window of the second half of pregnancy (Parnell et al., 2007). Arterial partial pressure of oxygen, however, did not change (Parnell et al., 2007). A major question remains: what is the driving force that links PAE-induced changes in CBF with developmental abnormalities? The answer is expected to be complex, considering that PAE triggers several changes in fetal metabolic parameters, and alcohol is a simple ligand that can simultaneously target many molecular entities.
Ethanol-driven alterations in fetal cerebrovascular function: persistent effects
Several groups have investigated whether the effect of PAE on fetal cerebrovascular function can still be observed at or after birth. Our group used Doppler ultrasonography on a baboon model of pregnancy to follow fetal cerebral artery development throughout gestation following three episodes of maternal alcohol intoxication (1.5 g/kg ethanol via oral gavage) at 90, 100 and 110 days of gestation (term at 175 days) (Tobiasz et al., 2017). While we observed a drop in fetal anterior and middle cerebral artery peak systolic velocity during maternal alcohol intoxication when maternal BAC reached 80 mg/dL, no difference in Doppler indices of these arteries between alcohol-exposed and control fetuses were observed at 135, 155 or 165 days of gestation (Tobiasz et al., 2017).
In non-primate species, persistent alterations of cerebrovascular function in physiology and pathology following PAE were documented. Earlier work in ovine model of pregnancy documented that PAE (1 g/kg ethanol i.v. maternal infusion for three weeks during first trimester equivalent) resulted in attenuated adaptive increase of CBF in response to hypoxia in 1–4 day-old term lambs (Gleason et al., 1997). As a result, oxygen delivery to the newborn brain could not be maintained. Thus, PAE as early as first trimester is capable of rendering cerebral vessels more vulnerable to environmental insult. In a more recent report, ovine adult cerebral artery dilatory responses to vasoactive intestinal peptide (VIP) were studied following PAE (Ngai et al., 2008). Penetrating intracerebral arterioles were harvested from the brains of adult (10–13 months old) sheep that were exposed to alcohol prenatally via maternal intravenous infusions of 1.5 g/kg ethanol during days 30–82 of gestation (term at 145–150 days). This ethanol infusion resulted in maternal BAC of over 200 mg/dL. Brain arterioles were dissected out and pressurized in vitro at 60 mm Hg. While there was no difference in myogenic tone between PAE and control groups, PAE significantly increased VIP-induced dilation. Notably, there was no difference in dilator responses to extraluminal acidosis (pH 6.8), or to a wide concentration range of adenosine or CGS21680 (an adenosine A2A receptor agonist) (Ngai et al., 2008). This fact is particularly interesting: when these compounds were probed on penetrating arterioles earlier, i.e., in the third trimester equivalent fetuses following a similar ethanol administration paradigm, PAE resulted in a larger dilatory response to micromolar concentrations of CGS21680 (Mayock et al., 2008). Thus, there seems to be a slow recovery from PAE-driven alterations in the pharmacological profile of cerebral arteries.
In addition to the ovine model, several reports in rodents document the long-lasting consequences of PAE. PAE of Sprague-Dawley rats via administration of a 3% ethanol liquid diet for the duration of the pregnancy led to attenuation of cerebral arteriole responses to endothelial and neuronal nitric oxide synthase-dependent pharmacological modulators at 4–6 weeks after birth (Cananzi and Mayhan, 2017). The response was recovered by the antioxidant apocynin, pointing at the critical role of reactive oxygen species in the vascular consequences of PAE (Cananzi and Mayhan, 2017).
In a mouse study, Bake et al. (2017) reported alterations in vascular function that lasted throughout adulthood (up to 12 months of age) following PAE. In this study, pregnant dams received a binge-like bolus of ethanol (3 g/kg) via intragastric gavage twice a day from 12.5 through 15.5 days of gestation (second trimester equivalent of pregnancy). This experimental paradigm resulted in a maternal peak BAC of 117 mg/dL (Bake et al., 2012). This fetal alcohol exposure resulted in selective alteration of the cranially directed blood flow through carotid arteries when compared to the function of renal and femoral arteries (Bake et al., 2017). A separate set of experiments was performed to evaluate the consequences of PAE on the recovery from stroke following the middle cerebral artery occlusion (MCAo) in 3-month-old mice. Twenty-four hours following MCAo, animals that were prenatally exposed to alcohol exhibited significantly greater motor deficit when compared to the control group, as was indicated by the post-stroke composite neurological score (Bake et al., 2017). Notably, diminished post-stroke recovery in the group of prenatally alcohol-exposed mice was not accompanied by an increased brain lesion size or increased stroke-induced cytokine production (Bake et al., 2017).
It is fair to conclude that while some of the alcohol-driven alterations of fetal cerebral artery pharmacological and physiological features vanish during development, others become persistent and could be observed later in development, even after birth.
Mechanistic insights into the pathophysiology of ethanol-driven alterations in fetal cerebrovascular function
Despite the translational value of the in vivo studies, these models cannot unequivocally address whether alcohol-driven changes in fetal CBF result from drug action on fetal neurovascular elements themselves or, rather, as a consequence of drug action on maternal hemodynamics. Indeed, alcohol consumption affects multiple parameters of maternal circulation, including systemic blood pressure and uteroplacental blood flow (Hill et al., 1983; Ramadoss and Magness, 2012). As a result, the observed effects of alcohol on fetal cerebrovascular function in vivo are likely to represent a composite of alcohol actions on fetal cerebrovascular networks themselves and on maternal hemodynamics. In addition, in vivo studies on animal models are often performed under anesthesia (such as during ethanol administration via gavage, or for the purpose of fetal Doppler evaluation). Functional consequences of ethanol interaction with anesthetics, including modulation of systemic and cerebral hemodynamics, are well described (Lichtor et al., 1993; El-Mas et al., 1994; Garfield and Bukusoglu, 1996; Luo et al., 2007). As a “conditioning” of ethanol effect by anesthetics cannot be ruled out, the use of anesthesia in studies of alcohol effect is considered to be a limitation of in vivo work (Parnell et al., 2007; Seleverstov et al., 2017; Tobiasz et al., 2017). Considering the aforementioned limitations of in vivo studies, in vitro work provides an opportunity to distinguish between maternal and fetal effects of alcohol and to remove the additional layers of complexity that result from using anesthesia. Moreover, experimentation in vitro allows discovery of molecular mechanisms that underlie PAE actions on fetal cerebrovascular function.
In a search for ethanol targets that enable widely reported increases in fetal CBF during maternal alcohol intoxication, our group used fetal baboons. Fetal MCA branches were harvested at the end of second trimester equivalent of human pregnancy and in vitro pressurized at 30 mmHg (Seleverstov et al., 2017). Consistent with in vivo studies that documented ethanol-drive increases in CBF, which were most likely driven by ethanol-induced dilation of cerebral vessels, our data showed that in vitro pressurized fetal cerebral arteries dilated in the presence of 63 mg/dL ethanol. This concentration was detected in the amniotic fluid of pregnant baboons during alcohol intoxication, with maternal BAC averaging 80 mg/dL (Seleverstov et al., 2017). Ethanol-induced dilation of fetal cerebral arteries was fully blunted by AM251 and AM630, cannabinoid receptor 1 and 2 blockers, respectively (Fig. 2). Although the critical role of the endocannabinoid (eCB) system in PAE-driven changes of neuronal function was reported (Basavarajappa, 2015), a study by Seleverstov et al. (2017) suggested the eCB system as a target of maternal alcohol consumption within the fetal cerebral artery. Moreover, upregulation of CB2 receptor-mediated cerebral artery dilation by anandamide was detected in fetuses at the end of second trimester following repeated alcohol exposure at gestational days 90, 100 and 110 (term at 175 days) (Seleverstov et al., 2017).
Figure 2. Short-term and long-lasting effects of prenatal alcohol exposure on fetal cerebral arteries.
Diagram summarizes molecular players that have been identified as targets of PAE in fetal cerebral arteries. Cerebral arteries contain three major layers. Cellular content of tunica intima (t.i.) is comprised of endothelial cells. Tunica media (t.m.) mostly contains vascular myocytes. The artery is covered by tunica adventitia (t.a.) that is rich in collagen. Fetal cerebral arteries dilate in the presence of alcohol, and this dilation is mediated by cannabinoid (CB) receptors 1 and 2. Distribution of CB receptors between fetal artery layers remains unclear. Persistent effects of PAE include upregulation of anandamide (AEA)-induced dilation, increased stiffness of the vessel that is accompanied by changes in collagen and tropoelastin mRNA levels, increased generation of reactive oxygen species (ROS), and downregulation of endothelial nitric oxide synthase (NOS) amount and function. 5-HT: 5-hydroxytriptamine.
Several mechanistic insights onto ethanol-driven alterations of fetal cerebral artery function are also offered from work on an ovine model of pregnancy (Parkington et al., 2014). In this study, ethanol at 0.75 g/kg was infused intravenously into pregnant ewes over 1 hour daily on gestational days 95 through 133, which represent late pregnancy (term at 145–150 days). Ethanol infusion resulted in 117 mg/dL maternal BAC and 108 mg/dL fetal plasma alcohol concentration (Parkington et al., 2014). Cerebral artery in vitro function was evaluated following fetal necropsy at 133 days of gestation (near term). Fetal cerebral artery constriction in response to KCl was similar in control and alcohol-exposed groups. Yet, vasodilation in response to the endothelium-dependent dilator 5-hydroxytriptamine was diminished in fetal cerebral arteries from alcohol-exposed donors. Moreover, PAE resulted in significant increases of fetal cerebral artery overall stiffness, as was evident by the nearly 2-fold increase in the artery elastic modulus in alcohol-exposed donors. Consistent with functional results, mRNA of endothelial nitric oxide synthase (eNOS) was significantly decreased while mRNA levels of collagen Ia1 and tropoelastin were significantly increased in the alcohol-exposed group when compared to control (Fig. 2). Notably, these changes occurred in the absence of detectable fetal growth restriction of alcohol-exposed fetuses (Parkington et al., 2014). This result further bolsters the theory that fetal cerebral arteries are highly sensitive to prenatal ethanol exposure, and react to alcohol exposure abruptly when compared to overall fetal growth parameter. The data by Parkington et al. (2014) are similar to the findings of our group showing that PAE-driven alterations in fetal cerebral artery function and morphometric parameters have different time-courses with changes in cerebral artery tone preceding those of fetal overall growth (Tobiasz et al., 2017). These results reiterate the idea that alterations in fetal cerebrovascular function may serve as a trigger of neurodevelopmental abnormalities that characterize FAS/FASD.
Ethanol-driven alterations in fetal microvessels
As found for major cerebral arteries and arterioles, the effect of PAE on the cerebral microvasculature has been studied in several animal models. In an ovine model of pregnancy, Simon et al. (2008) did not detect significant changes in cerebral microvessel density following binge alcohol exposure during the second trimester equivalent of human pregnancy. In this study, ewes received 1.5 g/kg of ethanol (1:3 dilution in saline for a total infusion volume of 5.7 mL/kg) via catheter in jugular vein. Ethanol was infused daily, with the exception of weekends, over a 1.5-hour interval. Ewes in the saline-control group received an equivalent volume of saline using the same protocol. Infusions were performed over a period of 25 days, beginning on day 60±2 of ovine gestation (term at 145–150 days). Maternal BAC in this protocol reached 216±20 mg/dL. Fetal brain microvessels were immunohistochemically labeled against endothelial nitric oxide synthase (eNOS), and their density was determined using unbiased stereology. The lack of effect of PAE during second trimester equivalent of human pregnancy in ovine model (Simon et al., 2008) contrasts with findings in rats following alcohol exposure on postnatal days 4 to 10 (Kelly et al., 1990). This postnatal age in rats corresponds to gestational weeks 36–40 (or term fetus) of human pregnancy based on the timing of neurobiology development indices (Semple et al., 2013). In the study by Kelly et al. (1990) the alcohol-exposed rats were given 6.6 g/kg of ethanol during eight hours of each 24 hour interval via a gastrostomy tube. Maternal BAC in this protocol reached 480±22.3 mg/dL. On postnatal day 10, capillary density and diameter were compared between cerebellum, hippocampus proper, and dentate gyrus. In cerebellum and hippocampus proper, there was no change in the capillary density, yet capillary diameters were increased as a result of alcohol exposure (Kelly et al., 1990). The results indicate that microvessel density and diameter in fetuses during term-equivalent of human pregnancy are sensitive to alcohol exposure (Fig. 3). The results by Kelly et al. (1990) differ from those of Simon et al. (2008), despite the fact that both groups used binge alcohol exposure protocol. Possible explanations for the discrepancy between the findings of these two studies include differences in timing of alcohol exposure (term versus second trimester equivalent of human pregnancy), the route of alcohol administration (gastrostomy versus i.v. infusion), maternal BAC that resulted from the alcohol exposure paradigm, and species difference.
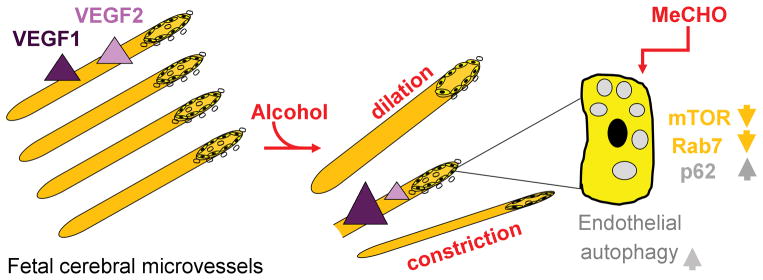
Figure 3. Modifications in fetal cerebral microvessels in response to prenatal alcohol exposure.
Diagram summarizes the effects of PAE on microvessel morphology and function. Microvessel dilation, constriction, and degeneration have all been reported as the consequences of PAE. PAE exposure upregulates receptor for vascular endothelial growth factor 1 (VEGF1), yet downregulates receptor for VEGF2. In addition, upregulation of endothelial autophagy following PAE has been reported. The increase in autophagy vacuole number following alcohol exposure is triggered by acetaldehyde (MeCHO) and is accompanied by a down-regulation of mammalian target of rapamycin (mTOR) pathway.
However, a profound effect of PAE on microvessel development and expression has been documented not only in sheep but also in rodents. In rats, 20–30 days old offspring that were prenatally exposed to alcohol still exhibit ultrastructural abnormalities in cortical capillaries (Popova, 1992). In a mouse model, the loss of radial orientation of the microvessels and a decrease in vascular density were both observed at postnatal day 2 (third trimester equivalent of human pregnancy; Patten et al., 2014) following daily subcutaneous injections of pregnant dams with 0.5, 1, or 3 g/kg ethanol administered from gestational day 13 to day 19 (Jégou et al., 2012). This time-line covers second trimester equivalent of human pregnancy (Patten et al., 2014). While alcohol in the maternal blood following injection of 0.5 g/kg ethanol was barely detectable, maternal BAC following injection of 1 and 3 g/kg ethanol resulted in averaged BAC of 60 and 200 mg/dL, respectively (Jégou et al., 2012). The lowest dose of injected alcohol (0.5 g/kg) did not have any effect on the microvessel density, while higher doses resulted in up to 30% decrease in vascular density within neocortex. This effect was accompanied by a significant loss in the radial orientation of the vessels. A similar result was observed in brain samples from FAS human donors at gestational weeks 30–38 (near-term to term; Patten et al., 2014) following microvessel staining with Glut1 (Jégou et al., 2012). Yet, there was no significant difference in microvessel histological characteristics between FAS and control groups of human donors of younger gestational age (19–30 weeks of gestation) (Jégou et al., 2012). This finding is in remarkable agreement with findings in animal models in which changes in microvessel morphological parameters are observed during third trimester equivalent (Kelly et al., 1990) of human pregnancy when compared to younger gestational age (Simon et al., 2008). Thus, the third trimester may represent a “window of vulnerability” to PAE with regard to morphological changes in cerebrovascular network. This finding is not surprising, as late gestation represents the timeframe during which microvessel networks undergo major development. In addition, a “window of vulnerability” for alcohol-driven neuronal loss in the developing brain was placed into the third trimester equivalent (Goodlett and Eilers, 1997; Thomas et al., 1998). The coinciding of microvessel and neuronal vulnerability to alcohol exposure further reinforces the importance of the vascular component in consequences of PAE for fetal brain.
Overall, available literature suggests that cerebral microvasculature responses to alcohol exposure are fine-tuned by multiple factors. For instance, in addition to showing disorientation of cerebral microvessels following PAE (see above), Jégou et al. (2012) used Western blots to demonstrate a significant increase in VEGF receptor type 1, but a decrease in VEGF receptor type 2 protein levels in cerebral vessels of PAE mouse donors. Acute treatment of mouse brain slices on postnatal day 2 with 25 to 100 mM ethanol resulted in a dose-dependent retraction of the cortical microvessels within 6 hours of monitoring. Moreover, slice perfusion with ethanol concentrations exceeding 50 mM led to a marked reduction in microvessel diameter and promoted vascular cell death (Jégou et al., 2012). The latter could be blocked with VEGF, thus, highlighting the role of VEGF system as a target of alcohol-driven pathology of fetal brain (Jégou et al., 2012).
A recent study by Girault et al. (2017) on human brain samples from alcohol-exposed fetuses and on the mouse model of PAE demonstrated that cerebral microvessel endothelium autophagy was impaired by ethanol. In particular, upregulation of autophagy vacuoles has been observed in human FAS fetal brain samples at 29–34 weeks of gestation. A similar result was obtained from mouse brain samples harvested at matching postnatal day 2 (Semple et al., 2013) following maternal subcutaneous daily injections with 3 g/kg ethanol from gestational day 13 to 19 (Girault et al., 2017). In mouse primary microvascular endothelial cells, the ethanol effect was ablated in presence of 4-methylpyrazole that blocks formation of ethanol metabolite acetaldehyde. The increase in autophagy vacuole numbers following ethanol treatment was linked to the inhibition of the mammalian target of rapamycin (mTOR) pathway, with rapamycin rescuing deleterious consequences of ethanol exposure. Moreover, ethanol inhibited p62 protein degradation within the lysosomes, thus reducing autophagy vacuole turnover. Finally, ethanol presence resulted in a mild but consistent decrease in Rab7, a protein that plays a critical role in endocytosis (Fig. 3) (Girault et al., 2017). Overall, while the exact role of endothelial cell autophagy in cerebral angiogenesis is still being established, ethanol-driven autophagy vacuole upregulation is likely to facilitate cell pathology or susceptibility to environmental insult. Thus, it is tempting to propose that ethanol-driven impairment of fetal cerebral microvessel endothelial cell autophagy is a mechanism that underlies well-documented abnormalities in cerebral angiogenesis following PAE (Girault et al., 2017). Moreover, rapamycin could be considered as a drug candidate for the prevention of ethanol toxicity associated with ethanol/acetaldehyde targeting of microvessel autophagy (Girault et al., 2017).
Blood-brain barrier (BBB) permeability has also been considered as a potential point of PAE-driven alteration within cerebral capillaries. In a study by Phillips et al. (1997), circulating horseradish peroxidase was used to trace the rat BBB permeability in response to dietary PAE. However, no evidence of ethanol-induced altering of capillary formation or BBB permeability was found (Phillips et al., 1997). Although cerebral microvessel leakage does not appear to play a major role in the pathophysiology of PAE, more studies are needed to elucidate the effect of alcohol on BBB permeability.
Concluding remarks
Numerous studies presented in this review unambiguously place the fetal cerebral circulation in the list of organs/systems that are severely affected by PAE. Several important aspects of fetal cerebrovascular alterations by PAE are already established. First, the effect of PAE on CBF and metabolic characteristics is generally concentration-dependent; second, alcohol-driven alterations in CBF are underlied by ethanol targeting of molecular players within the fetal cerebral blood vessel itself; and third, many manifestations of PAE in cerebral arteries and the microvessel network are persistent and have consequences far beyond the time limits of gestation. Finally, the aforementioned characteristics have been consistently observed in different animal models of PAE, reinforcing the universal nature of these findings.
Although several recent reports have advanced our understanding of PAE-driven alterations of the fetal cerebral circulation, several critical questions remain. How much of the neurological damage/deficit arises from direct neuronal targeting by alcohol versus neuronal damage by alcohol-driven alterations in cerebral blood flow? Can FAS/FASD manifestations be reversed or, at least, diminished by pharmacotherapeutic adjustment of fetal cerebrovascular alterations by alcohol? These questions are difficult to answer at this time given the complexity of cerebrovascular networks and scarce data on PAE’s actual targets themselves. Among the areas that require further clarification is the role of ethanol metabolites. For example, acetaldehyde has been shown to possess teratogenic properties on embryonic development and has long been considered as a contributor into the FAS/FASD etiology (Ryle and Thomson, 1983; Webster et al., 1983; Lee et al., 2005; VandeVoort et al., 2011). Recent work showing the critical role of acetaldehyde in ethanol-driven impairment of fetal cerebral microvessel autophagy (Girault et al., 2017) certainly revives interest in this compound and its reactions during prenatal development of cerebrovascular networks. On the other hand, further studies are needed to establish any contribution of BBB permeability to PAE-driven alterations of cerebral microvessel function. Studies into consequences of PAE on fetal neurovascular unit are also needed. Finally, it is critical to identify downstream molecular effectors of ethanol or its physiologically active metabolite(s) within fetal cerebrovascular components. This task presents an uphill battle: owing to the simplicity of its chemical structure, ethanol is a rather promiscuous chemical ligand. In addition to the multitude of proteins that are targeted by ethanol, mild amphiphilic properties allow ethanol penetration into biological membranes at large and the perturbation of their lipid constituents (Ho et al., 1994; Peoples et al., 1996; Liinamaa et al., 1997). Yet, the task is worth pursuing, as identification of intimate molecular mechanisms that produce deleterious consequences of PAE on fetal cerebral circulation will open new venues for development of effective treatments against FAS/FASD.
Acknowledgments
Authors deeply thank Ms. Patti Smith of the Office of Scientific Writing, Office of Research at UTHSC, for editorial assistance. This work was supported by NIH R21 AA-022433 (ANB), R01 AA-023764 (ANB), and R37 AA-11560 (AMD).
Abbreviations
- BAC
blood alcohol concentration
- BBB
blood-brain barrier
- BK
calcium-/voltage-gated potassium channel of large conductance
- CBF
cerebral blood flow
- CB1
cannabinoid (receptor) type 1
- CB2
cannabinoid (receptor) type 2
- DSC
dynamic susceptibility contrast (imaging)
- eCB
endocannabinoid (system)
- FAS
fetal alcohol syndrome
- FASD
fetal alcohol spectrum disorder
- MCA
middle cerebral artery
- MCAo
middle cerebral artery occlusion
- PAE
prenatal alcohol exposure
- PSS
physiologic sodium saline
- RyR
ryanodine receptor
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
- VIP
vasoactive intestinal peptide
Footnotes
Conflict of interest: none.
References
- Allsopp G, Gamble HJ. Light and electron microscopic observations on the development of the blood vascular system of the human brain. J Anat. 1979;128:461–477. [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Altura BT. Alcohol, the cerebral circulation and strokes. Alcohol. 1984;1:325–331. doi: 10.1016/0741-8329(84)90056-9. [DOI] [PubMed] [Google Scholar]
- Altura BM. Alcohol, stroke, hypertension and the heart. Introduction and overview. Alcohol. 1984;1:321–323. doi: 10.1016/0741-8329(84)90055-7. [DOI] [PubMed] [Google Scholar]
- Anthony B, Vinci-Booher S, Wetherill L, Ward R, Goodlett C, Zhou FC. Alcohol-induced facial dysmorphology in C57BL/6 mouse models of fetal alcohol spectrum disorder. Alcohol. 2010;44:659–671. doi: 10.1016/j.alcohol.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe PAE. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Bager H, Christensen LP, Husby S, Bjerregaard L. Biomarkers for the Detection of PAE: A Review. Alcohol Clin Exp Res. 2017;41:251–261. doi: 10.1111/acer.13309. [DOI] [PubMed] [Google Scholar]
- Bailey BA, Sokol RJ. Pregnancy and alcohol use: evidence and recommendations for prenatal care. Clin Obstet Gynecol. 2008;51:436–444. doi: 10.1097/GRF.0b013e31816fea3d. [DOI] [PubMed] [Google Scholar]
- Bakdash A, Burger P, Goecke TW, Fasching PA, Reulbach U, Bleich S, Hastedt M, Rothe M, Beckmann MW, Pragst F, Kornhuber J. Quantification of fatty acid ethyl esters (FAEE) and ethyl glucuronide (EtG) in meconium from newborns for detection of alcohol abuse in a maternal health evaluation study. Anal Bioanal Chem. 2010;396:2469–7710. doi: 10.1007/s00216-010-3474-5. [DOI] [PubMed] [Google Scholar]
- Bake S, Gardner R, Tingling JD, Miranda RC, Sohrabji F. Fetal Alcohol Exposure Alters Blood Flow and Neurological Responses to Transient Cerebral Ischemia in Adult Mice. Alcohol Clin Exp Res. 2017;41(1):117–127. doi: 10.1111/acer.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Tingling JD, Miranda RC. Ethanol exposure during pregnancy persistently attenuates cranially directed blood flow in the developing fetus: evidence from ultrasound imaging in a murine second trimester equivalent model. Alcohol Clin Exp Res. 2012;36:748–758. doi: 10.1111/j.1530-0277.2011.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Leeman L, Savich RD, Cano S, Gutierrez H, Savage DD, Rayburn WF. The validity of phosphatidylethanol in dried blood spots of newborns for the identification of PAE. Alcohol Clin Exp Res. 2014;38:1078–1085. doi: 10.1111/acer.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Savage DD. Focus on: biomarkers of fetal alcohol exposure and fetal alcohol effects. Alcohol Res Health. 2011;34:56–63. [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Nixon RA, Arancio O. Endocannabinoid system: emerging role from neurodevelopment to neurodegeneration. Mini Rev Med Chem. 2009;9:448–462. doi: 10.2174/138955709787847921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS. Fetal Alcohol Spectrum Disorder: Potential Role of Endocannabinoids Signaling. Brain Sci. 2015;5:456–493. doi: 10.3390/brainsci5040456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. The baboon (Papio sp.) as a model for female reproduction studies. Contraception. 2015;92:120–123. doi: 10.1016/j.contraception.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A. The endocannabinoid system in normal and pathological brain ageing. Philos Trans R Soc Lond B Biol Sci. 2012;367:3326–3341. doi: 10.1098/rstb.2011.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha M, Aaslid R, Douville CM, Correra R, Newell DW. Cerebral blood flow and dynamic cerebral autoregulation during ethanol intoxication and hypercapnia. J Clin Neurosci. 2003;10:195–198. doi: 10.1016/s0967-5868(02)00126-1. [DOI] [PubMed] [Google Scholar]
- Blood AB, Zhao Y, Long W, Zhang L, Longo LD. L-type Ca2+ channels in fetal and adult ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol. 2002;282:R131–R138. doi: 10.1152/ajpregu.00318.2001. [DOI] [PubMed] [Google Scholar]
- Bolognini D, Cascio MG, Parolaro D, Pertwee RG. AM630 behaves as a protean ligand at the human cannabinoid CB2 receptor. Br J Pharmacol. 2012;165:2561–2574. doi: 10.1111/j.1476-5381.2011.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew N, Walker D, Wong FY. Cerebral vascular regulation and brain injury in preterm infants. Am J Physiol Regul Integr Comp Physiol. 2014;306:R773–R786. doi: 10.1152/ajpregu.00487.2013. [DOI] [PubMed] [Google Scholar]
- Brien JF, Clarke DW, Richardson B, Patrick J. Disposition of ethanol in maternal blood, fetal blood, and amniotic fluid of third-trimester pregnant ewes. Am J Obstet Gynecol. 1985;152:583–590. doi: 10.1016/0002-9378(85)90632-5. [DOI] [PubMed] [Google Scholar]
- Bukiya AN, Vaithianathan T, Kuntamallappanavar G, Asuncion-Chin M, Dopico AM. Smooth muscle cholesterol enables BK β1 subunit-mediated channel inhibition and subsequent vasoconstriction evoked by alcohol. Arterioscler Thromb Vasc Biol. 2011;31:2410–2423. doi: 10.1161/ATVBAHA.111.233965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya A, Seleverstov O, Bisen S, Dopico A. Age-Dependent Susceptibility to Alcohol-Induced Cerebral Artery Constriction. JDAR. 2016;5:236002. doi: 10.4303/jdar/236002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd L, Klug MG, Li Q, Kerbeshian J, Martsolf JT. Diagnosis of fetal alcohol spectrum disorders: a validity study of the fetal alcohol syndrome checklist. Alcohol. 2010;44:605–614. doi: 10.1016/j.alcohol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Cananzi SG, Mayhan WG. In utero exposure to alcohol alters reactivity of cerebral arterioles. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17728163. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C, Wood E, Jabbour L. Impact of fetal alcohol exposure on body systems: A systematic review. Birth Defects Res C Embryo Today. 2016;108:174–180. doi: 10.1002/bdrc.21129. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Collen D. Vascular development and disorders: molecular analysis and pathogenic insights. Kidney Int. 1998;53:1519–1549. doi: 10.1046/j.1523-1755.1998.00936.x. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Molteno CD, Dodge NC, Meintjes EM, Jacobson SW. Fetal Alcohol Growth Restriction and Cognitive Impairment. Pediatrics. 2016;138:e20160775. doi: 10.1542/peds.2016-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Caprara D, Blanchette P, Klein J, Koren G. Recent developments in meconium and hair testing methods for the confirmation of gestational exposures to alcohol and tobacco smoke. Clin Biochem. 2004;37:429–3810. doi: 10.1016/j.clinbiochem.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Colvin L, Payne J, Parsons D, Kurinczuk JJ, Bower C. Alcohol consumption during pregnancy in nonindigenous west Australian women. Alcohol Clin Exp Res. 2007;31:276–284. doi: 10.1111/j.1530-0277.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- Drew PD, Kane CJ. Fetal alcohol spectrum disorders and neuroimmune changes. Int Rev Neurobiol. 2014;118:41–80. doi: 10.1016/B978-0-12-801284-0.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012;11:906–917. doi: 10.1016/S1474-4422(12)70135-7. [DOI] [PubMed] [Google Scholar]
- el-Mas MM, Tao S, Carroll RG, Abdel-Rahman AA. Ethanol-clonidine hemodynamic interaction in normotensive rats is modified by anesthesia. Alcohol. 1994;11:307–314. doi: 10.1016/0741-8329(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Ferreira MP, Willoughby D. Alcohol consumption: the good, the bad, and the indifferent. Appl Physiol Nutr Metab. 2008;33:12–20. doi: 10.1139/H07-175. [DOI] [PubMed] [Google Scholar]
- Flynn HA, Marcus SM, Barry KL, Blow FC. Rates and Correlates of Alcohol Use Among Pregnant Women in Obstetrics Clinics. Alcoholism: Clinical and Experimental Research. 2003 doi: 10.1097/01.ALC.0000046595.47491.37. [DOI] [PubMed] [Google Scholar]
- Fride E, Gobshtis N, Dahan H, Weller A, Giuffrida A, Ben-Shabat S. The endocannabinoid system during development: emphasis on perinatal events and delayed effects. Vitam Horm. 2009;81:139–158. doi: 10.1016/S0083-6729(09)81006-6. [DOI] [PubMed] [Google Scholar]
- Fride E. The endocannabinoid-CB(1) receptor system in pre- and postnatal life. Eur J Pharmacol. 2004;500:289–297. doi: 10.1016/j.ejphar.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Aguado T, Palazuelos J, Guzmán M. The endocannabinoid system and neurogenesis in health and disease. Neuroscientist. 2007;13:109–114. doi: 10.1177/1073858406296407. [DOI] [PubMed] [Google Scholar]
- Garfield JM, Bukusoglu C. Propofol and ethanol produce additive hypnotic and anesthetic effects in the mouse. Anesth Analg. 1996;83:156–161. doi: 10.1097/00000539-199607000-00027. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. 123I-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–338. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- Gauthier TW. PAE and the Developing Immune System. Alcohol Res. 2015;37:279–285. [PMC free article] [PubMed] [Google Scholar]
- Girault V, Gilard V, Marguet F, Lesueur C, Hauchecorne M, Ramdani Y, Laquerrière A, Marret S, Jégou S, Gonzalez BJ, Brasse-Lagnel C, Bekri S. Prenatal alcohol exposure impairs autophagy in neonatal brain cortical microvessels. Cell Death Dis. 2017;8:e2610. doi: 10.1038/cddis.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CA, Hotchkiss KJ. Cerebral responses to acute maternal alcohol intoxication in immature fetal sheep. Pediatr Res. 1992;31:645–648. doi: 10.1203/00006450-199206000-00021. [DOI] [PubMed] [Google Scholar]
- Gleason CA, Iida H, Hotchkiss KJ, Northington FJ, Traystman RJ. Newborn cerebrovascular responses after first trimester moderate maternal ethanol exposure in sheep. Pediatr Res. 1997;42:39–45. doi: 10.1203/00006450-199707000-00007. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21:738–744. [PubMed] [Google Scholar]
- Gordon N. Spontaneous intracranial hypotension. Dev Med Child Neurol. 2009;51:932–935. doi: 10.1111/j.1469-8749.2009.03514.x. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal R, Henderson DA, Chu N, Longo LD. Ovine middle cerebral artery characterization and quantification of ultrastructure and other features: changes with development. Am J Physiol Regul Integr Comp Physiol. 2012;302:R433–R445. doi: 10.1152/ajpregu.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Gutierrez HL, Hund L, Shrestha S, Rayburn WF, Leeman L, Savage DD, Bakhireva LN. Ethylglucuronide in maternal hair as a biomarker of PAE. Alcohol. 2015;49:617–623. doi: 10.1016/j.alcohol.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan N, Ben-Zvi A. The molecular, cellular, and morphological components of blood-brain barrier development during embryogenesis. Semin Cell Dev Biol. 2015;38:7–15. doi: 10.1016/j.semcdb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Heatley MK, Crane J. The blood alcohol concentration at post-mortem in 175 fatal cases of alcohol intoxication. Med Sci Law. 1990;30:101–105. doi: 10.1177/002580249003000203. [DOI] [PubMed] [Google Scholar]
- Heistad DH, Lawton WJ. Pathogenesis of acute hypertensive encephalopathy. In: Izzo JL, Black HR, editors. Hypertension Primer. 2. Lippincott Williams and Wilkins; Baltimore, MD: 1999. pp. 186–187. [Google Scholar]
- Hill DE, Slikker W, Jr, Goad PT, Bailey JR, Sziszak TJ, Hendrickx AG. Maternal, fetal, and neonatal elimination of ethanol in nonhuman primates. Dev Pharmacol Ther. 1983;6:259–268. doi: 10.1159/000457312. [DOI] [PubMed] [Google Scholar]
- Ho C, Williams BW, Kelly MB, Stubbs CD. Chronic ethanol intoxication induces adaptive changes at the membrane protein/lipid interface. Biochim Biophys Acta. 1994;1189:135–142. doi: 10.1016/0005-2736(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Ho R, Jacquemard R. Maternal Alcohol use before and during Pregnancy among Women in Taranaki and New Zealand. Journal of the New Zealand Medical Association. 2009;122:20–29. [PubMed] [Google Scholar]
- Humpel C. Chronic mild cerebrovascular dysfunction as a cause for Alzheimer’s disease? Exp Gerontol. 2011;46:225–232. doi: 10.1016/j.exger.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H, Gleason CA, O’Brien TP, Traystman RJ. Fetal responses to acute fetal cocaine injection in sheep. Am J Physiol. 1994;267:H1968–H1975. doi: 10.1152/ajpheart.1994.267.5.H1968. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Kaindl AM. Neuronal death and oxidative stress in the developing brain. Antioxid Redox Signal. 2011;14:1535–1550. doi: 10.1089/ars.2010.3581. [DOI] [PubMed] [Google Scholar]
- Jackson IT, Hussain K. Craniofacial and oral manifestations of fetal alcohol syndrome. Plast Reconstr Surg. 1990;85:505–512. doi: 10.1097/00006534-199004000-00003. [DOI] [PubMed] [Google Scholar]
- Jégou S, El Ghazi F, de Lendeu PK, Marret S, Laudenbach V, Uguen A, Marcorelles P, Roy V, Laquerrière A, Gonzalez BJ. Prenatal alcohol exposure affects vasculature development in the neonatal brain. Ann Neurol. 2012;72:952–960. doi: 10.1002/ana.23699. [DOI] [PubMed] [Google Scholar]
- Jimenez VA, Grant KA. Studies using macaque monkeys to address excessive alcohol drinking and stress interactions. Neuropharmacology. 2017;122:127–135. doi: 10.1016/j.neuropharm.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joya X, Friguls B, Ortigosa S, Papaseit E, Martínez SE, Manich A, Garcia-Algar O, Pacifici R, Vall O, Pichini S. Determination of maternal-fetal biomarkers of prenatal exposure to ethanol: a review. J Pharm Biomed Anal. 2012;69:209–222. doi: 10.1016/j.jpba.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Takebayashi S. The development of noradrenaline-, acetylcholinesterase-, neuropeptide Y- and vasoactive intestinal polypeptide-containing nerves in human cerebral arteries. Neurosci Lett. 1994;175:1–4. doi: 10.1016/0304-3940(94)91063-4. [DOI] [PubMed] [Google Scholar]
- Kanny D, Liu Y, Brewer RD, Lu H. Binge drinking — United States, 2011. MMWR Suppl. 2011;62:77–80. [PubMed] [Google Scholar]
- Kelly SJ, Mahoney JC, West JR. Changes in brain microvasculature resulting from early postnatal alcohol exposure. Alcohol. 1990;7:43–47. doi: 10.1016/0741-8329(90)90059-l. [DOI] [PubMed] [Google Scholar]
- Kenna K, Sozo F, De Matteo R, Hanita T, Gray SP, Tare M, Moritz K, Bertram JF, Black MJ, Brien JF, Parkington HC, Walker DW, Harding R. Alcohol exposure during late gestation: multiple developmental outcomes in sheep. J Dev Orig Health Dis. 2012;3:224–236. doi: 10.1017/S2040174412000244. [DOI] [PubMed] [Google Scholar]
- Klosowskii BM. The development of the Brain and its disturbance by harmful factors. Oxford: Pergamon Press; 1963. [Google Scholar]
- Knot HJ, Zimmermann PA, Nelson MT. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Castro C, Davis DM, Dudley D, Wey HY, Purdy D, Fox PT, Simerly C, Schatten G. Fetal brain during a binge drinking episode: a dynamic susceptibility contrast MRI fetal brain perfusion study. Neuroreport. 2010;21:716–721. doi: 10.1097/WNR.0b013e32833b5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017;171:948–956. doi: 10.1001/jamapediatrics.2017.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting CI, van Dommelen P, van der Pal-de Bruin KM, Bennebroek Gravenhorst J, van Wouwe JP. Prevalence and pattern of alcohol consumption during pregnancy in the Netherlands. BMC Public Health. 2015;15:723. doi: 10.1186/s12889-015-2070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- Lee RD, An SM, Kim SS, Rhee GS, Kwack SJ, Seok JH, Chae SY, Park CH, Choi YW, Kim HS, Cho HY, Lee BM, Park KL. Neurotoxic effects of alcohol and acetaldehyde during embryonic development. J Toxicol Environ Health A. 2005;68:2147–2162. doi: 10.1080/15287390500177255. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Thomas KG, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW. Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2015;39:724–732. doi: 10.1111/acer.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtor JL, Zacny JP, Coalson DW, Flemming DC, Uitvlugt A, Apfelbaum JL, Lane BS, Thisted RA. The interaction between alcohol and the residual effects of thiopental anesthesia. Anesthesiology. 1993;79:28–35. doi: 10.1097/00000542-199307000-00007. [DOI] [PubMed] [Google Scholar]
- Liinamaa MJ, Hannuksela ML, Kesäniemi YA, Savolainen MJ. Altered transfer of cholesteryl esters and phospholipids in plasma from alcohol abusers. Arterioscler Thromb Vasc Biol. 1997;17:2940–2947. doi: 10.1161/01.atv.17.11.2940. [DOI] [PubMed] [Google Scholar]
- Lin MT, Hessinger DA, PAErce WJ, Longo LD. Modulation of BK channel calcium affinity by differential phosphorylation in developing ovine basilar artery myocytes. Am J Physiol Heart Circ Physiol. 2006;291:H732–H740. doi: 10.1152/ajpheart.01357.2005. [DOI] [PubMed] [Google Scholar]
- Lintonen T, Ahlström S, Metso L. The reliability of self-reported drinking in adolescence. Alcohol Alcohol. 2004;39:362–368. doi: 10.1093/alcalc/agh071. [DOI] [PubMed] [Google Scholar]
- Liput DJ, Pauly JR, Stinchcomb AL, Nixon K. Binge Alcohol Exposure Transiently Changes the Endocannabinoid System: A Potential Target to Prevent Alcohol-Induced Neurodegeneration. Brain Sci. 2017;7:E158. doi: 10.3390/brainsci7120158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Garland M, Duan Y, Stark RI, Xu D, Dong Z, Bansal R, Peterson BS, Kangarlu A. Study of the development of fetal baboon brain using magnetic resonance imaging at 3 Tesla. Neuroimage. 2008;40:148–159. doi: 10.1016/j.neuroimage.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Xi Q, Ahmed A, Jaggar JH, Dopico AM. Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci U S A. 2004;101:18217–18222. doi: 10.1073/pnas.0406096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long W, Zhang L, Longo LD. Cerebral artery sarcoplasmic reticulum Ca(2+) stores and contractility: changes with development. Am J Physiol Regul Integr Comp Physiol. 2000;279:R860–R873. doi: 10.1152/ajpregu.2000.279.3.R860. [DOI] [PubMed] [Google Scholar]
- Long W, Zhang L, Longo LD. Fetal and adult cerebral artery K(ATP) and K(Ca) channel responses to long-term hypoxia. J Appl Physiol (1985) 2002;92:1692–1701. doi: 10.1152/japplphysiol.01110.2001. [DOI] [PubMed] [Google Scholar]
- Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J Pediatr. 1979;94:118–121. doi: 10.1016/s0022-3476(79)80373-x. [DOI] [PubMed] [Google Scholar]
- Luo F, Li Z, Treistman SN, Kim YR, King JA, Fox GB, Ferris CF. Confounding effects of volatile anesthesia on CBV assessment in rodent forebrain following ethanol challenge. J Magn Reson Imaging. 2007;26:557–563. doi: 10.1002/jmri.21083. [DOI] [PubMed] [Google Scholar]
- Madelaine R, Sloan SA, Huber N, Notwell JH, Leung LC, Skariah G, Halluin C, Paşca SP, Bejerano G, Krasnow MA, Barres BA, Mourrain P. MicroRNA-9 Couples Brain Neurogenesis and Angiogenesis. Cell Rep. 2017;20:1533–1542. doi: 10.1016/j.celrep.2017.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manich A, Velasco M, Joya X, García-Lara NR, Pichini S, Vall O, García-Algar O. Validity of a maternal alcohol consumption questionnaire in detecting prenatal exposure. An Pediatr (Barc) 2012;76:324–328. doi: 10.1016/j.anpedi.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Mann LI, Bhakthavathsalan A, Liu M, Makowski P. Effect of alcohol on fetal cerebral function and metabolism. Am J Obstet Gynecol. 1975;122:845–851. doi: 10.1016/0002-9378(75)90726-7. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Maternal risk factors for fetal alcohol spectrum disorders: not as simple as it might seem. Alcohol Res Health. 2011;34:15–26. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- Mayock DE, Ngai AC, Mondares RL, Gleason CA. Effects of binge alcohol exposure in the second trimester on intracerebral arteriolar function in third trimester fetal sheep. Brain Res. 2008;1226:111–115. doi: 10.1016/j.brainres.2008.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy FP, O’Keeffe LM, Khashan AS, North RA, Poston L, McCowan LM, Baker PN, Dekker GA, Roberts CT, Walker JJ, Kenny LC. Association between maternal alcohol consumption in early pregnancy and pregnancy outcomes. Obstet Gynecol. 2013;122:830–837. doi: 10.1097/AOG.0b013e3182a6b226. [DOI] [PubMed] [Google Scholar]
- Menshawi K, Mohr JP, Gutierrez J. A Functional Perspective on the Embryology and Anatomy of the Cerebral Blood Supply. J Stroke. 2015;17:144–158. doi: 10.5853/jos.2015.17.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan DW. Failure of autoregulation and intraventricular haemorrhage in preterm infants. Lancet. 1980;1:896–898. doi: 10.1016/s0140-6736(80)90836-3. [DOI] [PubMed] [Google Scholar]
- Moody WJ. Critical periods of early development created by the coordinate modulation of ion channel properties. Perspect Dev Neurobiol. 1995;2:309–315. [PubMed] [Google Scholar]
- Morley R, Dwyer T, Hynes KL, Cochrane J, Ponsonby AL, Parkington HC, Carlin JB. Maternal alcohol intake and offspring pulse wave velocity. Neonatology. 2010;97:204–211. doi: 10.1159/000252973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Moore EM, Thomas JD, Riley EP. Advances in Diagnosis and Treatment of Fetal Alcohol Spectrum Disorders: From Animal Models to Human Studies. Alcohol Res. 2015;37:97–108. [PMC free article] [PubMed] [Google Scholar]
- Ngai AC, Mondares RL, Mayock DE, Gleason CA. Fetal alcohol exposure alters cerebrovascular reactivity to vasoactive intestinal peptide in adult sheep. Neonatology. 2008;93:45–51. doi: 10.1159/000105524. [DOI] [PubMed] [Google Scholar]
- O’Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50:1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier L, Curfs LM, Viljoen DL. Fetal alcohol spectrum disorders: Prevalence rates in South Africa. S Afr Med J. 2016;106:S103–S106. doi: 10.7196/SAMJ.2016.v106i6.11009. [DOI] [PubMed] [Google Scholar]
- Palmer A, Klein R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev. 2003;17:1429–1450. doi: 10.1101/gad.1093703. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Kenna KR, Sozo F, Coleman HA, Bocking A, Brien JF, Harding R, Walker DW6, Morley R, Tare M. Maternal alcohol consumption in pregnancy enhances arterial stiffness and alters vasodilator function that varies between vascular beds in fetal sheep. J Physiol. 2014;592:2591–603. doi: 10.1113/jphysiol.2013.262873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, Ramadoss J, Delp MD, Ramsey MW, Chen WJ, West JR, Cudd TA. Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions: ovine model. Exp Physiol. 2007;92:933–943. doi: 10.1113/expphysiol.2007.038091. [DOI] [PubMed] [Google Scholar]
- Patten AR, Fontaine CJ, Christie BR. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front Pediatr. 2014;2:93. doi: 10.3389/fped.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- PAErce W. Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol (1985) 2006;100:731–738. doi: 10.1152/japplphysiol.00990.2005. [DOI] [PubMed] [Google Scholar]
- Peoples RW, Li C, Weight FF. Lipid vs protein theories of alcohol action in the nervous system. Annu Rev Pharmacol Toxicol. 1996;36:185–201. doi: 10.1146/annurev.pa.36.040196.001153. [DOI] [PubMed] [Google Scholar]
- Perlman JM, McMenamin JB, Volpe JJ. Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med. 1983;309:204–209. doi: 10.1056/NEJM198307283090402. [DOI] [PubMed] [Google Scholar]
- Phillips DE, Krueger SK, Wall KA, Smoyer-Dearing LH, Sikora AK. The development of the blood-brain barrier in alcohol-exposed rats. Alcohol. 1997;14:333–343. doi: 10.1016/s0741-8329(96)00180-2. [DOI] [PubMed] [Google Scholar]
- Pierce DR, West JR. Blood alcohol concentration: a critical factor for producing fetal alcohol effects. Alcohol. 1986;3:269–272. doi: 10.1016/0741-8329(86)90036-4. [DOI] [PubMed] [Google Scholar]
- Pillay P, Manger PR. Order-specific quantitative patterns of cortical gyrification. Eur J Neurosci. 2007;25:2705–2712. doi: 10.1111/j.1460-9568.2007.05524.x. [DOI] [PubMed] [Google Scholar]
- Popova EN. Prenatal moderate effects of alcohol on ultrastructure of cortical capillaries in the offspring. Biull Eksp Biol Med. 1992;113:161–164. [PubMed] [Google Scholar]
- Popova S, Lange S, Probst C, Gmel G, Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e290–e299. doi: 10.1016/S2214-109X(17)30021-9. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Vascular effects of maternal alcohol consumption. Am J Physiol Heart Circ Physiol. 2012;303:H414–H421. doi: 10.1152/ajpheart.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre JA, Hurt H. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120:e1245–e1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Raun BM, Kristensen NB. Metabolic effects of feeding ethanol or propanol to postpartum transition Holstein cows. J Dairy Sci. 2011;94:2566–2580. doi: 10.3168/jds.2010-3999. [DOI] [PubMed] [Google Scholar]
- Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. 2003;289:579–588. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Patrick JE, Bousquet J, Homan J, Brien JF. Cerebral metabolism in fetal lamb after maternal infusion of ethanol. Am J Physiol. 1985;249:R505–R509. doi: 10.1152/ajpregu.1985.249.5.R505. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryle PR, Thomson AD. Acetaldehyde and the fetal alcohol syndrome. Lancet. 1983;2:219–220. doi: 10.1016/s0140-6736(83)90199-x. [DOI] [PubMed] [Google Scholar]
- Santhosh D, Huang Z. Regulation of the nascent brain vascular network by neural progenitors. Mech Dev. 2015;138:37–42. doi: 10.1016/j.mod.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher MS. Developmental origins of cerebrovascular disease I: prenatal cerebrovascular development--classic findings in the context of advances in genetic and fetal surveillance evaluations. J Child Neurol. 2012;27:121–131. doi: 10.1177/0883073811417714. [DOI] [PubMed] [Google Scholar]
- Sierra S, Luquin N, Navarro-Otano J. The endocannabinoid system in cardiovascular function: novel insights and clinical implications. Clin Auton Res. 2017 doi: 10.1007/s10286-017-0488-5. in press. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Pallarés M, Nadal R, Ferré N. Opposite effects of ethanol and ketamine in the elevated plus-maze test in Wistar rats undergoing a chronic oral voluntary consumption procedure. J Psychopharmacol. 2002;16:305–312. doi: 10.1177/026988110201600404. [DOI] [PubMed] [Google Scholar]
- Simon KE, Mondares RL, Born DE, Gleason CA. The effects of binge alcohol exposure in the 2nd trimester on the estimated density of cerebral microvessels in near-term fetal sheep. Brain Res. 2008;1231:75–80. doi: 10.1016/j.brainres.2008.06.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Savory J, Couves J, Burns E. Alcohol consumption during pregnancy: cross-sectional survey. Midwifery. 2014;30:1173–1178. doi: 10.1016/j.midw.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Solonskii AV, Logvinov SV, Kutepova NA. Development of brain vessels in human embryos and fetuses in conditions of prenatal exposure to alcohol. Neurosci Behav Physiol. 2008;38:373–376. doi: 10.1007/s11055-008-0053-8. [DOI] [PubMed] [Google Scholar]
- Stewart CB, Disotell TR. Primate evolution - in and out of Africa. Curr Biol. 1998;8:R582–R588. doi: 10.1016/s0960-9822(07)00367-3. [DOI] [PubMed] [Google Scholar]
- Tabrizchi R, Pang CC. Influence of intravenous infusion of ethanol on regional blood flow in conscious rats. J Pharm Pharmacol. 1993;45:151–153. doi: 10.1111/j.2042-7158.1993.tb03704.x. [DOI] [PubMed] [Google Scholar]
- Tan CO, Hamner JW, Taylor JA. The role of myogenic mechanisms in human cerebrovascular regulation. J Physiol. 2013;591:5095–5105. doi: 10.1113/jphysiol.2013.259747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh NX, Jonsson E. Drinking Alcohol during Pregnancy: Evidence from Canadian Community Health Survey 2007/2008. J Popul Ther Clin Pharmacol. 2010;17:e302–e307. [PubMed] [Google Scholar]
- Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Brain Res Dev Brain Res. 1998;105:159–166. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- Tobiasz AM, Duncan JR, Bursac Z, Sullivan RD, Tate DL, Dopico AM, Bukiya AN, Mari G. The Effect of PAE on Fetal Growth and Cardiovascular Parameters in a Baboon Model of Pregnancy. Reprod Sci. 2017;1:1933719117734317. doi: 10.1177/1933719117734317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SH, Tew JM, Shipley MT. Cerebral arterial innervation: II. Development of calcitonin-gene-related peptide and norepinephrine in the rat. J Comp Neurol. 1989;279:1–12. doi: 10.1002/cne.902790102. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Carretero-Ortega J, Menéndez J, Narvaez C, Sun B, Lancaster E, Pershad V, Trzaska S, Véliz E, Kamei M, Prendergast A, Kidd KR, Shaw KM, Castranova DA, Pham VN, Lo BD, Martin BL, Raible DW, Weinstein BM, Torres-Vázquez J. Reck enables cerebrovascular development by promoting canonical Wnt signaling. Development. 2016;143:147–159. doi: 10.1242/dev.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeVoort CA, Hill DL, Chaffin CL, Conley AJ. Ethanol, acetaldehyde, and estradiol affect growth and differentiation of rhesus monkey embryonic stem cells. Alcohol Clin Exp Res. 2011;35:1534–1540. doi: 10.1111/j.1530-0277.2011.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgintino D, Errede M, Robertson D, Girolamo F, Masciandaro A, Bertossi M. VEGF expression is developmentally regulated during human brain angiogenesis. Histochem Cell Biol. 2003;119:227–232. doi: 10.1007/s00418-003-0510-y. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Suarez J, Llorente-Berzal A, López-Gallardo M, de Fonseca FR. The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. J Psychopharmacol. 2012;26:164–176. doi: 10.1177/0269881111408956. [DOI] [PubMed] [Google Scholar]
- Vutskits L. Cerebral blood flow in the neonate. Paediatr Anaesth. 2014;24:22–29. doi: 10.1111/pan.12307. [DOI] [PubMed] [Google Scholar]
- Wang YC, Chuang YH, Shao Q, Chen JF, Chen SY. Brain cytoplasmic RNA 1 suppresses smooth muscle differentiation and vascular development in mice. J Biol Chem. 2018 doi: 10.1074/jbc.RA117.001578. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kroenke CD. Utilization of Magnetic Resonance Imaging in Research Involving Animal Models of Fetal Alcohol Spectrum Disorders. Alcohol Res. 2015;37:39–51. [PMC free article] [PubMed] [Google Scholar]
- Webster WS, Walsh DA, McEwen SE, Lipson AH. Some teratogenic properties of ethanol and acetaldehyde in C57BL/6J mice: implications for the study of the fetal alcohol syndrome. Teratology. 1983;27:231–243. doi: 10.1002/tera.1420270211. [DOI] [PubMed] [Google Scholar]
- Wagerle LC, Kurth CD, Roth RA. Sympathetic reactivity of cerebral arteries in developing fetal lamb and adult sheep. Am J Physiol. 1990;258:H1432–H1438. doi: 10.1152/ajpheart.1990.258.5.H1432. [DOI] [PubMed] [Google Scholar]
- Wilhoit LF, Scott DA, Simecka BA. Fetal Alcohol Spectrum Disorders: Characteristics, Complications, and Treatment. Community Ment Health J. 2017;53:711–718. doi: 10.1007/s10597-017-0104-0. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Cudd TA. Focus on: the use of animal models for the study of fetal alcohol spectrum disorders. Alcohol Res Health. 2011;34:92–98. [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Jian K, Jaggar JH, Bukiya AN, Dopico AM. Type 2 ryanodine receptors are highly sensitive to alcohol. FEBS Lett. 2014;588:1659–1665. doi: 10.1016/j.febslet.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MK, Parsa AT, Horton JC. Skull thickening, paranasal sinus expansion, and sella turcica shrinkage from chronic intracranial hypotension. J Neurosurg Pediatr. 2011;11:667–672. doi: 10.3171/2013.2.PEDS12560. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Xiao H, Long W, PAErce WJ, Longo LD. Expression of several cytoskeletal proteins in ovine cerebral arteries: developmental and functional considerations. J Physiol. 2004;558:623–632. doi: 10.1113/jphysiol.2004.064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurolo E, Iyer AM, Spliet WG, Van Rijen PC, Troost D, Gorter JA, Aronica E. CB1 and CB2 cannabinoid receptor expression during development and in epileptogenic developmental pathologies. Neuroscience. 2010;170:28–41. doi: 10.1016/j.neuroscience.2010.07.004. [DOI] [PubMed] [Google Scholar]