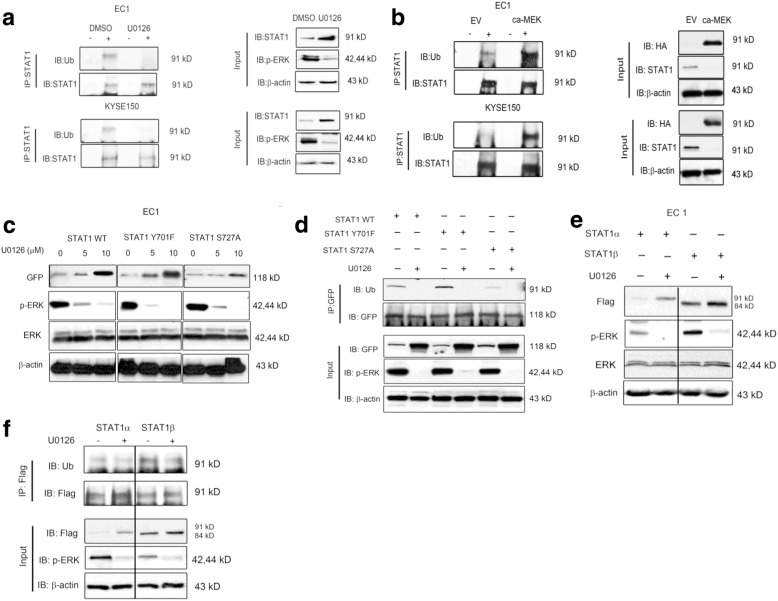
Fig. 2.
ERK promotes polyubiquitination of STAT1 independent of STAT1 phosphorylation. a. Immunoprecipitation experiments were performed to evaluate the level of ubiquitination of STAT1 in EC1 and KYSE150 cells treated with or without U0126 for 2 h. b. HA-ca-MEK plasmid was transfected into both ESCC cell lines together with empty vector (E.V.). STAT1 ubiquitination was detected by immunoprecipitation with anti-STAT1 antibody and immunoblotting with an anti-Ub antibody. c. GFP-STAT1 (WT), GFP-STAT1 (Y701F), or GFP-STAT1 (S727A) were transfected into EC1 cells together with increasing amounts of U0126. The protein level of GFP, p-ERK and ERK in the lysates was determined by Western blot. d. GFP-STAT1 (WT), GFP-STAT1 (Y701F), or GFP-STAT1 (S727A) were transfected into EC1 cells together with or without U0126. STAT1 ubiquitination was detected by immunoprecipitation with anti-GFP antibody and immunoblotting with anti-Ub antibody. e. Flag-STAT1α and Flag-STAT1β were transfected into EC1 together with or without U0126. The protein level of Flag, p-ERK and ERK in the lysates was determined by Western blot. f. Flag-STAT1α and Flag-STAT1β were transfected into EC1 with or without U0126. STAT1 ubiquitination was detected by immunoprecipitation with anti-Flag antibody and immunoblotting with anti-Ub antibody. Similar results were observed in three independent experiments