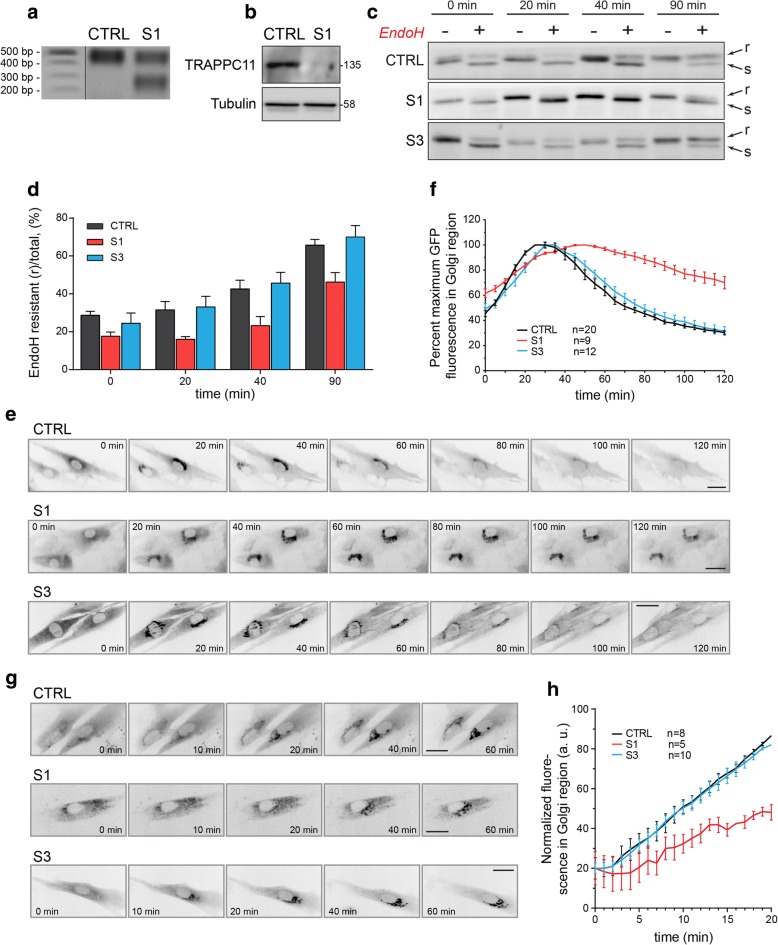
Fig. 3.
TRAPPC11 compound heterozygous mutations affect membrane trafficking in patient fibroblasts. a mRNA was collected from control and subject 1 (S1), converted to cDNA and amplified by PCR using oligonucleotides annealing to exons 8 and 11. The amplicons were sequenced and found to represent exons 8-9-10-11 (higher molecular size amplicon) and exons 8-part of 10-11 (lower molecular size amplicon). b Lysates from control and subject 1 (S1) fibroblasts were probed for TRAPPC11 and tubulin as a loading control. c Fibroblasts were infected with VSVG-GFP ts045, and the protein was arrested in the endoplasmic reticulum (ER) by shifting the cells to 40 °C. The protein was synchronously released from the ER upon downshifting the temperature to 32 °C, and the acquisition of Endoglycosidase H (EndoH) resistance was assayed at the times indicated. A representative western blot is displayed, and quantification of a minimum of three such blots is shown in d. e The same assay as in b was performed on live cells and the arrival and release of the GFP signal was quantified over time. Representative images from the movies are displayed in e, and quantification of the signal in the Golgi region is shown in f. To more accurately measure ER-to-Golgi trafficking, the RUSH assay [36] was performed using ST-SBP-GFP with the Ii hook (g). Images were acquired over time in live cells upon addition of biotin to initiate release of the protein from the ER. Quantification of the signal in the Golgi is displayed in h. Size bars in e and g denote 25 μm. Error bars represent SEM from a minimum of three replicates in d. N values for f and h are indicated in the figure