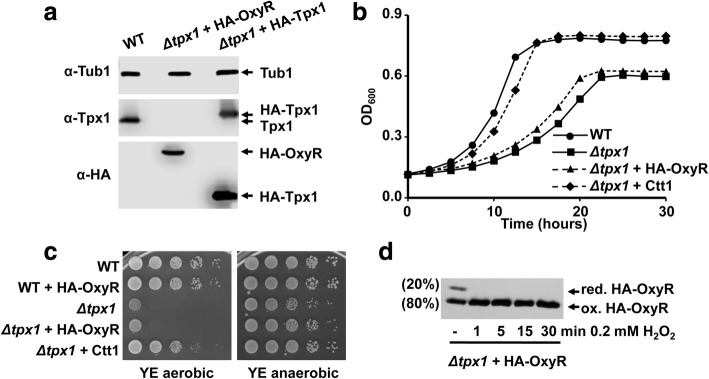
Fig. 4.
OxyR acts as a H2O2 sensor but not as a H2O2 scavenger. a Quantification of the intracellular concentration of OxyR expressed in fission yeast. TCA/AMS protein extracts were obtained from untreated MM cultures of strains 972 (WT), AD36 (Δtpx1) carrying an integrative sty1 promoter-driven HA-oxyR gene, and SG5 (Δtpx1) carrying an episomal 41× nmt promoter-driven HA-tpx1 gene (p123.41×), and processed by SDS-PAGE and analyzed by Western blot using antibodies against HA or Tpx1. Anti-tubulin was used as a loading control (Tub1). b,c Overexpression of OxyR cannot suppress the growth defects of cells lacking Tpx1, while catalase can. b Serial dilutions of YE cultures of strains 972 (WT), AD29 (WT + HA-OxyR), SG4 (Δtpx1), AD36 (Δtpx1 + HA-OxyR), and AD7 (Δtpx1 + Ctt1) were spotted on YE agar plates and grown for 3 days at 30 °C under anaerobic or aerobic conditions. Both HA-OxyR and catalase were overexpressed to similar levels from integrative plasmids with the sty1 promoter. c Growth of YE cultures of strains as in (b) was monitored by recording OD600 for a period of 30 h at 30 °C. d Determination of the steady-state levels of f4:5 intracellular H2O2 in cells lacking Tpx1 based on OxyR oxidation. MM cultures of AD36 (Δtpx1 expressing HA-OxyR) were treated or not with 0.2 mM f4:6 H2O2 for the times indicated, and TCA/AMS extracts were obtained and analyzed as described in Fig. 1b. The percentage of reduced (red.) and oxidized f4:7 (ox.) HA-OxyR in untreated. AMS 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid, MM minimal medium, ox. oxidized, red. reduced, SDS-PAGE sodium dodecyl sulphate-polyacrylamide gel electrophoresis, TCA trichloroacetic acid, WT wild type, YE rich medium, OD600 optical density at 600 nm