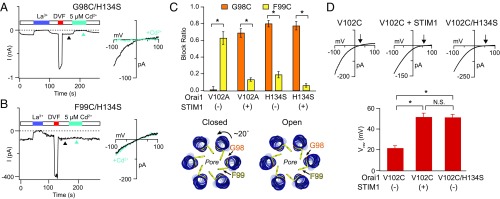
Fig. 4.
Cd2+ accessibility studies suggest that the pore conformation of H134S Orai1 is similar to that of STIM1-gated channels. (A and B) Cd2+ (5 µM) strongly blocks G98C, but not F99C, residues in the H134S mutant. I–V relationships were measured at times indicated by the arrowheads. (C) Summary of Cd2+ blockade at G98C or F99C in the H134S mutant (n = 4–6 cells; values are mean ± SEM). For comparison, Cd2+ blockade at these positions is also illustrated in the constitutively conducting V102A channels in the presence or absence of STIM1 (data from ref. 11). The pattern of blockade in the H134S mutant resembles that seen in STIM1-bound V102A channels, suggesting similar pore conformations. A schematic illustrating the open and closed states of the Orai1 pore showing rotation of the pore helix induced by STIM1 binding (adapted from ref. 46). (D) I–V relationships of the V102C mutant alone, or in the presence of either STIM1 or an additional H134S mutation. The lower graph summarizes the Vrev under these conditions. As seen with STIM1, H134S confers Ca2+ selectivity to V102C channels. Arrowheads indicate reversal potentials (n = 6–9 cells; values are mean ± SEM; *P < 0.05).