Fig. 7.
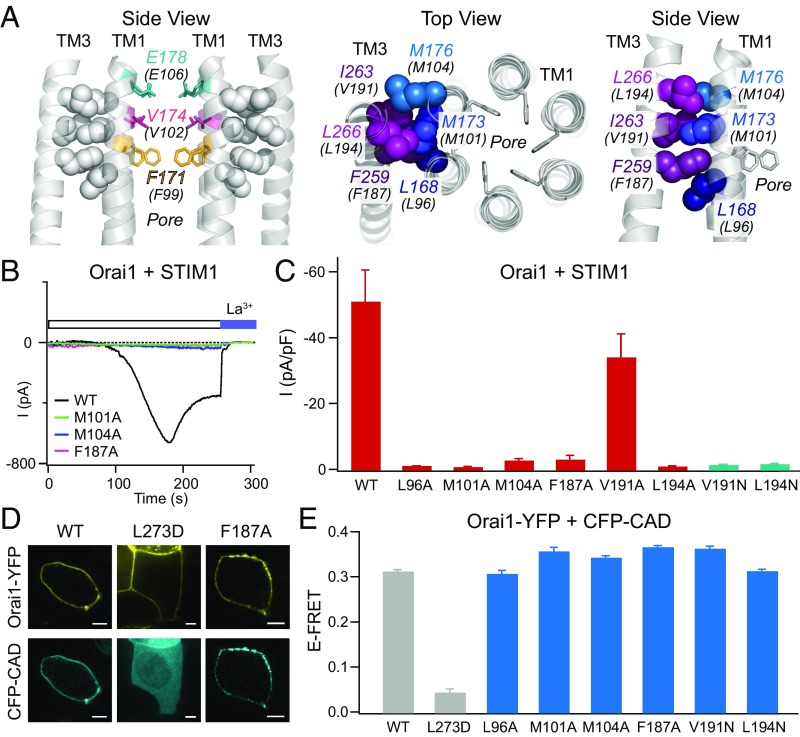
The TM3–TM1 hydrophobic cluster is essential for Orai1 gating by STIM1. (A) (Left) Side view of the hydrophobic contacts (gray spheres) between the TM1 pore helices with the TM3 segment. The selectivity filter (E106, teal) and hydrophobic gate residues (V102, magenta; F99, yellow) are also shown. Four TM1 helices and two TM3 helices are shown for simplicity. (Middle and Right) Top-down and side views of the six residues forming the hydrophobic stack. Amino acids are labeled with dOrai numbering with hOrai1 numbering shown in parentheses. (B) Point mutations of the hydrophobic stack abrogate Orai1 activation by STIM1. Representative traces of WT Orai1 and M101A, M104A, and F187A Orai1 mutants coexpressed with STIM1 following whole-cell break-in are shown. (C) Current densities of the indicated Orai1 mutations in the TM3–TM1 hydrophobic cluster coexpressed with STIM1 (n = 4–6 cells; values are mean ± SEM). With the exception of V191A, these mutations abrogate Orai1 activation by STIM1. (D) Confocal images of WT, L273D, and F187A Orai1-YFP constructs coexpressed with CFP-CAD showing normal CAD binding in the F187A mutant. (Scale bars: 5 μm.) (E) FRET between the indicated Orai1-YFP channels and CFP-CAD shows that the LOF mutations in the hydrophobic stack do not impair Orai1-STIM1 binding (n = 53–88 cells for each mutant; values are mean ± SEM).