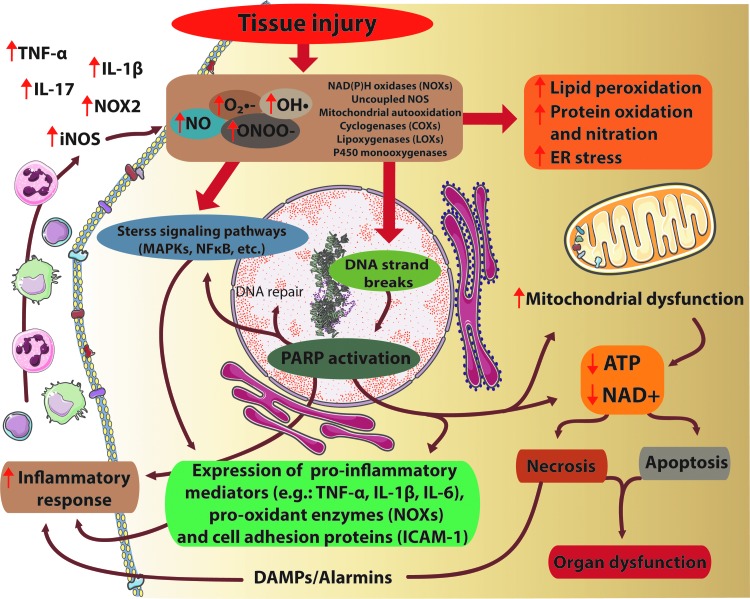
FIG. 1.
Interplay of oxidative/nitrative stress, inflammation, and cell death pathways in tissue injury. Tissue injury ultimately results in excessive O2•−, OH• production by NADPH oxidases (NOXs), uncoupled nitric oxide synthase (NOS) enzymes, mitochondrial autooxidation, lipoxigenases, or by the P450 monooxygenase system. Furthermore, in the presence of NO, the reactive free radical peroxynitrite (ONOO−) can form as well. All these agents cause massive cell damage, lipid peroxidation, mitochondrial damage, endoplasmic reticulum (ER) stress, impaired calcium handling, and nitration of tyrosine residue of different proteins. Tyrosine nitration disturbs the structure of proteins, alterations in the catalytic activity of enzymes, and impairs cell signaling pathways. Moreover, ONOO− -induced DNA damage and the subsequent overactivation of the NAD+-consuming poly(APD-ribose) polymerase-1 (PARP-1) enzyme compromise energy homeostasis. These detrimental effects of excessive formation of reactive oxygen/nitrogen species (ROS/RNS) ultimately lead to energy failure, neurotoxicity and neuronal death. On the other hand, ROS can enhance the formation of proinflammatory and pro-oxidant proteins by stimulation of MAPK or NFκB pathways, resulting in enhanced expression of proinflammatory mediators, facilitating the inflammatory response, leukocyte recruitment that contributes to an enhanced NO production by inducible NOS (iNOS), and further aggravation of ROS production. MAPK, mitogen-activated protein kinase; NAD+, nicotinamide adenine dinucleotide; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; O2•−, superoxide; OH•, hydroxyl radical. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars