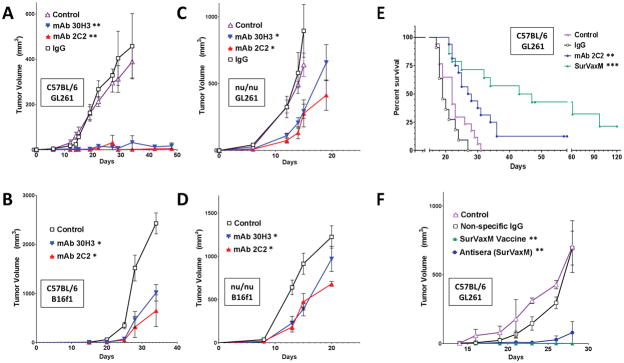
Figure 5.
Subcutaneous (A–D and F) and intracranial (E) tumor models in C57BL/6 (A, B, E and F) and nude (C and D) mice with GL261 glioma (A, C, E and F), and B16f1 melanoma (B and D) cells. Mice were given the indicated treatments once every 5 days beginning 3 days following tumor cell implantation (A, C n = 8–10 per group; B, D n = 5 per group). Subcutaneous tumors (A–D and F) were measured and tumor volumes were calculated as described. (E) Mice with GL261 intracranial tumors were treated with PBS (control), nonspecific IgG, mAb 2C2 at a dose of 10 or 20 μg, or SurVaxM vaccine (n = 11–17 per group) and survival was determined by the Kaplan-Meier method. Median survival time and range; control=22 days (range 17–31 days), nonspecific IgG = 19 days (range 17–27 days), mAb 2C2 = 28 days (range 21–58+ days), SurVaxM = 45 days (range 21–120+ days). (F) Mice with GL261 flank tumors were treated with conjugated survivin vaccine (SVN53-67/M57-KLH; SurVaxM), pooled antiserum derived from non-tumor-bearing mice that had been vaccinated with SurVaxM, or nonspecific IgG control antibody (n = 4 per group). Statistical significance in A–D and F was assessed using the Wilcoxon matched-pairs test; *p<0.05; **p<0.0014. Statistical significance in E was assessed using the Logrank Mantel-Cox test **p=0.0041 and ***p<0.0001.