Figure 2. Venetoclax/GDC-0980 inactivates AKT/mTOR/p70S6K, down-regulates MCL-1, triggers BAX mitochondrial translocation, and effectively kills primary AML blasts including CD34+/38−/123+ AML progenitor but not normal CD34+ cells.
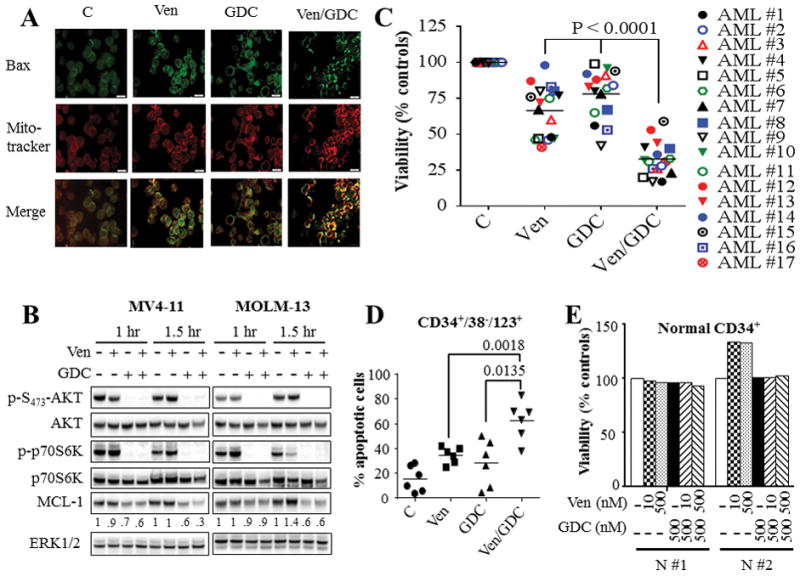
(A) MV4-11 cells were exposed to 10 nM venetoclax (Ven) and/or 500 nM GDC-0980 (GDC) for 2 hr after which BAX translocation to the mitochondria was assessed using IX71 Olympus microscope. (B) MV4-11 and MOLM-13 cells were treated with 10 nM venetoclax (Ven) and/or 500 nM GDC-0980 (GDC) for the indicated intervals, after which cells were lysed and the lysates were subjected to western blot analysis. Densitometry analysis was performed on MCL-1 blots using Image Studio lite Software (Li-Cor Biosciences), and values were normalized for ERK1/2 loading controls. (C) Assessment of cell viability using Annexin V/7-AAD or CellTiter-Glo luminescent assays for 17 primary AML specimens with a preponderance of blasts (≥ 80%) following 16 hr treatment with venetoclax and GDC-0980 alone or together. As in the case of cell lines, primary AML samples exhibited heterogeneous responses to venetoclax. Concentrations of venetoclax were selected based upon marginal toxicity when administered alone and clinical relevance. Venetoclax concentrations varied between 10 – 100 nM for patients #1–10, and between 200 – 2000 nM for patients #11 – 17. GDC-0980 was administered at concentrations varying between 200 nM and 1000 nM. The median values for combined treatment were significantly lower than values for either agent alone (P < 0.0001 in each case). (D) Primary AML blasts from 6 patients were treated as in (C) for 16 hr after which the extent of the cell death was assessed selectively in AML progenitor CD34+/CD38−/CD123+ cells by Annexin V/7-AAD staining. The median values were significantly lower for combined treatment compared to either agent alone (P < 0.0018; P < 0.0135 for venetoclax and GDC-0980 respectively). (E) Normal hematopoietic mononuclear cells were isolated from umbilical cord blood of 2 subjects (N #1 and N #2) and were exposed to the designated concentrations of venetoclax and GDC-0980 alone or in combination for 16 hr after which viability was assessed selectively in the CD34+ cell population by Annexin V/7-AAD staining.
