Figure 4. Functional roles of BAX, BAK, and BIM in venetoclax activity in vitro and in vivo.
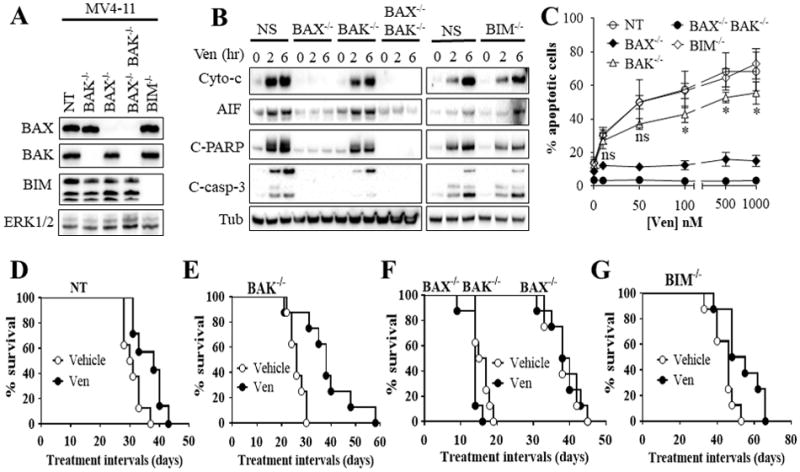
(A) western blot analysis in untreated MV4-11 cells in which BAX (BAX−/−), BAK (BAK−/−), double BAX/BAK (BAX−/−BAK−/−), or BIM (BIM−/−) were knocked out using CRISPR technology as described in Methods or non-targeting (NT) control cells. (B) These cell were exposed to 100 nM venetoclax (Ven) for 2 or 6 hr after which cytosolic fractions were isolated and subjected to western blot analysis. (C) Cells were treated with the designated concentrations of venetoclax for 24 hr after which the extent of apoptosis was determined using Annexin V staining. Error Bars, S.D for at least 3 independent experiments.*, P < 0.05; ns = non-significant for BAK−/− versus NT cells. Values obtained for BAX−/− or BAX−/−BAK−/− cells before versus after venetoclax treatment were not significantly different, P > 0.05 in each case. (D–G) NOD/SCID-γ mice were inoculated with 3 × 106 BAX−/−, BAK−/−, BAX−/−BAK−/−, BIM−/−, or NT control MV4-11 cells via tail vein. One week later, mice were treated orally with 75 mg/kg venetoclax (Ven) once every day, 6 days a week. Mouse survival was analyzed by Kaplan-Meier survival plot. Survival of mice with Bax−/− or Bax−/−Bak−/−-derived xenografts are both included in Figure 4F. These studies involved 8 mice/condition. Venetoclax significantly prolonged mouse survival in mice with NT, BAK−/−, or BIM−/− cells, P = 0.0138, P = 0.0015, and P = 0.0117 respectively. In contrast, no survival benefit was observed in mice with BAX−/− or BAX−/−BAK−/− cells.
