Abstract
Purpose
We examined whether short-term exposure to in vitro maturation (IVM) medium of cumulus-oocyte complexes (COCs) from a stimulated cycle increases the yield of metaphase II (MII) oocytes and usable embryos.
Methods
Retrospective review of two consecutive autologous IVF/ICSI cycles per patient between 2007 and 2015 in which cycle 1 did not result in live birth. Patients with short-term exposure of COCs to IVM medium (3–5 h before standard insemination or ICSI) in cycle 2 (treated) were matched 1:4 on %MI and %MII to patients without use of IVM in cycle 2 (untreated). The proportions of mature oocytes, two pronucleate (2PN) zygotes, number of usable embryos, and clinical outcomes were compared between groups with regression modeling.
Results
The treated (n = 43) and untreated (n = 163) groups had similar demographic characteristics and similarly high proportions of immature oocytes (48.2 vs. 41.3%, respectively) in cycle 1. There were no significant differences between the treated and untreated groups in the change in %MII (48.1 to 68.9% vs. 50.5 to 72.5%, respectively) or mean number of usable embryos (2.2 to 3.4 vs. 2.0 to 3.3, respectively) from cycle 1 to cycle 2.
Conclusions
These findings suggest that short-term IVM incubation of COCs may not provide any additional benefit in patients with a prior unsuccessful cycle notable for a high proportion of immature oocytes. Further randomized studies are warranted to determine whether there is a subset of patients who may have improved clinical outcomes with this “rescue IVM” intervention.
Keywords: In vitro maturation, Oocytes, IVF, Maturity
Introduction
Despite the classic definition of in vitro maturation (IVM) as the maturation of immature oocytes in vitro after their recovery from follicles not previously exposed to either exogenous LH or hCG prior to retrieval, exposure of oocytes to IVM medium of varying duration has now been studied in the context of stimulated cycles in attempts to increase mature oocyte yield. Although pregnancies from immature oocytes retrieved from stimulated cycles and exposed to IVM medium were reported as early as 1997 [1], the clinical efficacy of this intervention has not been well established. Furthermore, prior literature has varied in its terminology and methodology when referring to the exposure of oocytes to IVM medium, making outcomes for variations of IVM medium exposure difficult to compare [2–5]. Importantly, the various uses of “IVM” have recently been discussed and clarified so as to distinguish the classic definition of IVM from the looser applications of the term currently used in clinical IVF [5].
Typically, 70–85% of oocytes from stimulated cycles are mature (metaphase II, MII stage), with the remainder variably at prophase I (the germinal vesicle, GV stage) or metaphase I (MI stage) [6, 7]. Interestingly, some women tend to produce high numbers of immature oocytes (> 30% oocyte cohort) despite use of contemporary stimulation and trigger regimens for clinical IVF. In this challenging population, the yield of two pronucleate (2PN) zygotes, fertilization, and implantation rates are typically low [8, 9]. The underlying pathophysiology of this condition is not fully understood, but abnormal follicular response during growth and potential defects in nuclear and/or cytoplasmic maturation in response to the ovulatory trigger likely play a role [10–12].
While completion of meiotic maturation from prophase I to MII requires approximately 36 h, the time for oocytes retrieved as MI to progress to MII after controlled ovarian stimulation appears to take 3–5 h [4, 6, 10, 13, 14]. Attempts have therefore been made to “rescue” oocytes retrieved at the MI stage by exposure of these oocytes to IVM medium. De Vos and colleagues compared fertilization rate and embryo quality in embryos from MII oocytes injected immediately after retrieval versus those derived from cumulus-free MI oocytes matured after 4 h in culture [3]. Despite a lower fertilization rate from the IVM group, the authors concluded that short-term exposure to IVM medium may be beneficial for women with a lower yield of mature oocytes after ovarian stimulation because good quality embryos could be obtained from the IVM cohort, and one live birth was achieved [3]. However, other studies, with the limitation of differing methodologies, have raised questions regarding the overall efficacy of IVM on the normality of mitochondrial distribution, cleavage rates, implantation rates, gene expression, and ploidy [2, 4, 15–18].
Despite these potential shortcomings of IVM, rigorous investigation is lacking of the potential benefit of using short-term exposure of all retrieved COCs to IVM medium among patients with a prior unsuccessful cycle and high proportion of immature oocytes. Therefore, for several years, our program has adopted the policy to use this application of IVM in patients who had a prior unsuccessful IVF cycle often with a high proportion of immature oocytes in the hopes of improving mature oocyte yield in this challenging population. The aim of the present study was therefore to test the hypothesis that short-term exposure of COCs to IVM medium prior to ICSI improves laboratory and clinical outcomes in patients with a prior unsuccessful IVF cycle by increasing the incidence of mature oocytes and usable embryos.
Methods
IRB approval
This retrospective study was approved by the Partners’ Healthcare Institutional Review Board.
Study design
This study included all patients undergoing autologous IVF or ICSI cycles at Brigham and Women’s Hospital (BWH) between 2007 and 2015 with two cycles occurring within 1 year (cycles 1 and 2), in which cycle 1 did not result in a live birth. In cycle 2, the treated group included all women who were selected by their provider to undergo short-term exposure to IVM medium immediately after oocyte retrieval (treated group). These women were typically referred to this treatment due to a high (> 30%) proportion of immature oocytes. Those untreated underwent conventional IVF/ICSI (untreated group) with a similar duration of incubation in standard medium before insemination or preparation for ICSI. The treated group was matched to the untreated group by %MI and %MII (both within 5%) in cycle 1; most cycles (n = 34) were matched 1:4, with the remainder (n = 9) matched 1:3. The cycle outcomes of patients in the treated group were compared both to their prior unsuccessful cycle and to outcomes in the matched untreated population.
The following cycles were excluded in entirety: gestational carrier cycles, long-term banking cycles, split IVF/ICSI insemination, and donor cycles. PGD/PGS cycles were included for analyses of laboratory outcome data, but excluded from analyses of clinical outcomes.
Clinical protocols
Standard controlled ovarian stimulation was performed, including luteal down-regulation with leuprolide acetate (Lupron; TAP Pharmaceuticals, Lake Forest, IL), GnRH antagonist protocols with or without estradiol priming, microflare, or ovulation induction (OI) per clinical indication as previously described [9, 19–21]. Cycle monitoring was performed with serial estradiol measurements and transvaginal ultrasound to assess follicular development. When the two lead follicles reached a mean diameter of at least 18 mm, urinary or recombinant hCG or a dual GnRH agonist-hCG trigger was administered for final oocyte maturation with retrieval occurring 36–37 h later. Luteal support was achieved with either IM progesterone (25–100 mg/d, locally compounded at one of two pharmacies: Village Fertility, Waltham, MA or Freedom Fertility, Byfield, MA) starting 1 day post-retrieval, or vaginal progesterone once daily (Crinone 8%, Watson Pharmaceuticals, Parsippany, NJ, USA), starting 2 days post-retrieval. Support was continued until a negative pregnancy test or 10-week gestation.
Laboratory protocols
When short-term exposure to IVM medium was not performed (i.e., for cycles 1 and 2 of the untreated group and cycle 1 of the treated group), COCs were inseminated 4–6 h, or injected within 3–5 h of egg retrieval as described previously [19, 22, 23]. Prior to ICSI, COCs were exposed to 80 IU/mL hyaluronidase (Sigma Aldrich, St. Louis, MO, USA) for 30 to 60 s and then mechanically denuded using 150-μm stripper pipettes (MidAtlantic Diagnostics Inc., Mount Laurel, NJ, USA). Oocyte meiotic status was assessed after hyaluronidase exposure and cumulus-corona cell removal and prior to injection in cases of ICSI and at the fertilization check in conventional IVF; oocytes were classified as GV stage, MI, or MII. Only those oocytes at MII were injected.
Whether inseminated or injected, oocytes were assessed for pronuclei status at the fertilization check 16–18 h later. Zygotes with 2PN were incubated in 25 μL drops of growth medium [G1.2, G1.3 (Scandinavian IVF Science, Gothenburg, Sweden/Vitrolife, Englewood, CO, USA) or Global Total (Life Global, CT, USA)] and overlain with 8 mL equilibrated mineral oil (Scandinavian IVF Science, Gothenburg, Sweden/Vitrolife, Englewood, CO, USA) in Falcon 1007 culture dishes (Becton Dickinson Labware, Franklin Lakes, NJ, USA). Gamete and embryo cultures were maintained at 37 °C either in a humidified atmosphere of 5% CO2 in air (1/1/2007–5/24/2012) or in an un-humidified atmosphere of 5% O2, 6–7% CO2 and balance of N2 (from 5/25/12 to present). On day 3, embryos were graded for cell number, fragmentation (scores 0 through 4), and symmetry (scores 1 through 3) using standard criteria as previously described [24]. Good quality embryos were defined as those with a cell number greater or equal to 8, fragmentation score of 0 or 1, and symmetry score of 1 or 2.
Short-term exposure to IVM medium (i.e., in cycle 2 of the treated group) consisted of the intact COCs being placed in SAGE IVM medium (Cooper Surgical, Trumbull, CT, USA) immediately after retrieval and subsequently incubated for 3–5 h [9]. The IVM medium was supplemented with 75 mIU/mL FSH and 75 mIU/mL LH. Following this short-term IVM exposure, handling of gametes was identical to that of the untreated group.
Outcomes
The primary outcomes compared the treated and untreated groups for changes from cycle 1 to cycle 2 in %MI, %MII, and usable embryo yield (i.e., percentage of those embryos that were transferred or frozen). Other outcomes assessed included the total number of oocytes retrieved, number and % GVs, number of MI and MII oocytes, and number and % of 2PNs. Clinical outcomes included implantation rate (number of gestational sacs/number embryos transferred) and, of those cycles resulting in a transfer, the rates of chemical/ectopic pregnancy, clinical pregnancy (presence of gestational sac), graduation to obstetrical care (i.e., having a viable fetus at ≥ 7 weeks), and live birth.
Statistical analysis
Using SAS (9.3), logistic and linear regressions comparing cycle 2 outcomes between the treated and untreated groups were adjusted for patient age, stimulation protocol (antagonist, Lupron down-regulation, microflare or estradiol priming, or OI), type of trigger (hCG 5000 IU, hCG 10,000 IU, hCG/Lupron or Lupron vs. Ovidrel 250 mcg or Ovidrel 500 mcg), number of hours from trigger to retrieval, and whether ICSI was used. A linear regression was also performed to compare the change in embryology outcomes across cycles between the treated and untreated groups. A Kruskal-Wallis test was used to compare distributions of embryo cell numbers between the groups. A Poisson regression was used to compare implantation rates. P values < 0.05 were considered statistically significant.
Results
Demographic, clinical, and cycle characteristics
The comparison groups comprised 43 patients, who underwent conventional IVF/ICSI in cycle 1 and short-term exposure of COCs to IVM medium in cycle 2, and 163 patients who did not undergo IVM medium exposure in either cycle 1 or cycle 2. The demographic characteristics and cycle characteristics of these two groups of patients are shown in Tables 1 and 2, respectively. The mean ages and BMIs of the women in the untreated and treated groups at the time of cycle 1 were similar. More Asian women tended to be referred for IVM in cycle 2 (18.6 vs. 6.1% in the untreated group). The most common infertility diagnoses were male factor, unexplained, and diminished ovarian reserve with the treated group having a higher proportion of women with unexplained infertility (treated vs. untreated 34.9 vs. 24.5% in cycle 1; 37.2 vs. 24.5% in cycle 2, Table 1). AMH levels were on average higher in the untreated group in cycle 1 and cycle 2 (4.3 and 4.4 ng/mL, respectively, vs. 2.7 and 2.6 ng/mL in the treated group). The stimulation protocols, trigger types, peak estradiol levels, gonadotropin dosing, and days of stimulation were similar across groups in each cycle (Table 2). There were minimal differences in times from trigger to egg retrieval, hyaluronidase, and ICSI between the groups (Table 2).
Table 1.
Baseline demographic characteristics of the untreated and IVM treated groups (n = 206)
| Untreated group | IVM treated group+ | |||
|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | |
| No IVM (n = 163) | No IVM (n = 163) | No IVM (n = 43) | With IVM (n = 43) | |
| Mean age (years)* | 36.8 ± 4.3 | 37.3 ± 4.3 | 36.4 ± 4.4 | 36.9 ± 4.4 |
| Mean BMI (kg/m2)** | 25.6 ± 6.2 | 25.4 ± 6.1 | 25.9 ± 5.8 | 26.0 ± 5.8 |
| < 18.5 | 7 (4.4) | 7 (4.3) | 1 (2.3) | 1 (2.4) |
| 18.5–24.9 | 89 (56.0) | 94 (57.7) | 22 (51.2) | 21 (50.0) |
| 25.0–29.9 | 32 (20.1) | 31 (19.0) | 10 (23.3) | 10 (23.8) |
| 30.0–39.9 | 24 (15.1) | 24 (14.7) | 8 (18.6) | 8 (19.1) |
| ≥ 40 | 7 (4.4) | 7 (4.3) | 2 (4.7) | 2 (4.8) |
| Race/ethnicity** | ||||
| Caucasian | 112 (68.7) | 113 (69.3) | 21 (48.8) | 22 (51.2) |
| Black or African-American | 10 (6.1) | 10 (6.1) | 4 (9.3) | 4 (9.3) |
| Asian | 10 (6.1) | 10 (6.1) | 7 (16.3) | 8 (18.6) |
| Hispanic | 8 (4.9) | 8 (4.9) | 1 (2.3) | 1 (2.3) |
| Other | 3 (1.8) | 3 (1.8) | 1 (2.3) | 1 (2.3) |
| Unknown | 20 (12.2) | 19 (11.7) | 9 (21.0) | 8 (18.6) |
| Diagnosis **++ | ||||
| Tubal factor | 19 (11.7) | 20 (12.3) | 1 (2.3) | 1 (2.3) |
| Uterine factor | 7 (4.3) | 7 (4.3) | 1 (2.3) | 1 (2.3) |
| Endometriosis | 11 (6.8) | 11 (6.8) | 1 (2.3) | 1 (2.3) |
| Ovulatory | 17 (10.4) | 17 (10.4) | 3 (6.7) | 2 (4.7) |
| DOR | 47 (28.8) | 49 (30.1) | 8 (18.6) | 9 (20.9) |
| Unexplained | 40 (24.5) | 40 (24.5) | 15 (34.9) | 16 (37.2) |
| Male | 51 (31.3) | 50 (30.7) | 16 (37.2) | 16 (37.2) |
| Other | 27 (16.6) | 28 (17.2) | 5 (11.6) | 5 (11.6) |
| Gravid | 73 (44.8) | 85 (52.2) | 25 (58.1) | 24 (55.8) |
| Parous | 34 (20.9) | 34 (20.9) | 9 (20.9) | 9 (20.9) |
| D3 FSH (mIU/mL)* | 9.0 ± 5.5 | 9.2 ± 6.0 | 8.2 ± 4.2 | 7.8 ± 3.6 |
| D3 estradiol (pg/mL)* | 42.1 ± 22.8 | 40.7 ± 23.1 | 34.2 ± 23.2 | 34.4 ± 23.0 |
| AMH (ng/mL)* | 4.3 ± 7.7 | 4.4 ± 7.7 | 2.7 ± 2.1 | 2.6 ± 2.1 |
| Cycle number* | 1.2 ± 0.5 | 2.2 ± 0.5 | 2.0 ± 1.1 | 3.0 ± 1.1 |
+Defined by women who undergo IVM in cycle 2
*Values are mean ± SD
**Vales are number (% total)
++Some women possess multiple diagnoses and thus, these values do not add to 100%
Table 2.
Cycle characteristics of the untreated group and IVM treated group
| Cycle characteristic | Untreated group | IVM treated group | ||
|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | |
| No IVM (n = 163) | No IVM (n = 163) | No IVM (n = 43) | With IVM (n = 43) | |
| Stimulation protocol* | ||||
| Antagonist | 40 (24.5) | 44 (27.0) | 12 (27.9) | 18 (41.9) |
| Lupron down-regulation | 79 (48.5) | 50 (30.7) | 20 (46.5) | 13 (30.2) |
| Microflare or estradiol priming | 36 (22.1) | 69 (42.3) | 9 (20.9) | 12 (27.9) |
| Ovulation induction | 8 (4.9) | 0 | 2 (4.7) | 0 |
| Peak estradiol, pg/mL** | 1843.0 ± 857.4 | 1959.5 ± 867.5 | 2016.3 ± 863.6 | 2204.5 ± 1053.3 |
| Total LH dose, IU | 1022.4 ± 1339.9 | 1554.8 ± 1167.6 | 1008.1 ± 1097.9 | 1384.9 ± 1079.4 |
| Total FSH dose, IU | 3141.7 ± 2055.4 | 3293.1 ± 1904.9 | 2829.1 ± 1881.9 | 3184.9 ± 1825.5 |
| Days of stimulation | 12.7 ± 3.1 | 12.6 ± 2.4 | 11.9 ± 2.12 | 12.6 ± 1.9 |
| Number follicles measured** | ||||
| < 12 mm | 10.2 ± 5.1 | 10.7 ± 5.1 | 12.1 ± 4.1 | 12.6 ± 6.1 |
| 12–15 mm | 5.7 ± 3.9 | 6.1 ± 4.1 | 7.9 ± 4.1 | 7.4 ± 4.9 |
| 16–17 mm | 2.0 ± 1.9 | 2.1 ± 1.6 | 1.9 ± 1.14 | 2.3 ± 1.7 |
| ≥ 18 mm | 2.5 ± 1.5 | 2.6 ± 1.6 | 2.3 ± 1.2 | 2.9 ± 1.4 |
| Trigger type* | ||||
| hCG 5000 | 0 (0) | 3 (1.8) | 0 (0) | 2 (4.7) |
| hCG 10,000 | 140 (85.9) | 141 (86.5) | 37 (86.1) | 38 (88.4) |
| hCG/lupron | 3 (1.8) | 5 (3.1) | 0 | 0 |
| Lupron | 1 (0.6) | 0 (0) | 0 | 0 |
| Ovidrel 250 mcg | 14 (8.6) | 10 (6.1) | 5 (11.6) | 3 (7.0) |
| Ovidrel 500 mcg | 5 (3.1) | 4 (2.5) | 1 (2.3) | 0 |
| ICSI* | 82 (50.3) | 112 (68.7) | 25 (58.1) | 34 (79.1) |
| Time from trigger to retrieval (h)** | 36.1 ± 0.5 | 36.3 ± 0.5 | 36.2 ± 0.6 | 36.81 ± 0.6 |
| Time from trigger to hyaluronidase (h)** | 41.2 ± 1.1 | 41.27 ± 1.0 | 41.8 ± 1.1 | 42.63 ± 1.1 |
| Time from retrieval to ICSI (h)** | 5.06 ± 1.0 | 4.93 ± 1.1 | 5.43 ± 0.9 | 5.85 ± 1.1 |
*Values are number (% total)
**Values are mean ± SD
Oocyte yield and maturity and usable embryo yield
Oocyte yield and maturity, and proportion of usable embryos among all four cycles, with corresponding regression coefficients comparing cycle 2 outcomes, are shown in Table 3. Total oocyte yield ranged from 10 to 14 oocytes among the four cycles. The % immature oocytes (GV + MI) retrieved was similar between treated and untreated groups in cycle 1 (48.2 ± 15.9 vs. 41.3 ± 18.6%, respectively). There was no significant difference between the decrease from cycle 1 to cycle 2 in %MI oocytes observed in the treated and untreated groups (15.9% decrease 23.9 ± 17.6 to 8.0 ± 9.4% vs. 11.6% decrease 23.2 ± 17.4 to 11.6 ± 13.6%, respectively).
Table 3.
Embryology outcomes
| Untreated group | IVM treated group | Linear regression β (95% CI)++ | Linear regression β (95% CI)++** | |||
|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | |||
| No IVM (n = 163) | No IVM (n = 163) | No IVM (n = 43) | With IVM (n = 43) | |||
| Total number oocytes retrieved* | 10.4 ± 6.3 | 10.8 ± 7.2 | 14.2 ± 6.7 | 12.9 ± 6.5 | 1.95 (− 0.43,4.32) | 1.17 (− 1.37,3.71) |
| % GV+/total oocytes | 18.1 ± 17.4 | 13.0 ± 17.0 | 24.3 ± 17.6 | 17.5 ± 17.2 | 4.64 (− 1.13,10.40) | 1.14 (− 4.65,6.94) |
| % MI+/total oocytes | 23.2 ± 17.4 | 11.6 ± 13.6 | 23.9 ± 17.6 | 8.0 ± 9.4 | − 3.66 (− 8.01,0.69) | − 4.43 (− 9.18,0.32) |
| % Immature oocytes (GV and MI)+/total oocytes | 41.3 ± 18.6 | 24.5 ± 21.0 | 48.2 ± 15.9 | 25.5 ± 19.5 | 1.00 (−6.03,7.98) | −3.28 (−10.58,4.01) |
| No. MII* | 5.5 ± 4.2 | 7.6 ± 5.2 | 7.0 ± 4.5 | 8.5 ± 4.6 | 0.87 (− 0.82,2.56) | 0.71 (− 1.07,2.48) |
| % MII+/total oocytes | 50.5 ± 14.8 | 72.5 ± 21.7 | 48.1 ± 15.7 | 68.9 ± 20.3 | − 3.87 (− 11.12,3.38) | 1.26 (− 6.7,8.80) |
| % Other oocytes, any oocyte not a GV, MI or MII+ | 8.1 ± 15.0 | 3.0 ± 7.6 | 4.7 ± 6.7 | 5.7 ± 9.1 | 2.89 (0.22,5.57) | 2.02 (− 0.90,4.94) |
| No. 2PN* | 3.1 ± 2.9 | 5.4 ± 4.2 | 4.1 ± 3.4 | 6.3 ± 3.9 | 0.90 (− 0.50,2.30) | 0.72 (− 0.76,2.21) |
| % 2PN/MII | 59.0 ± 32.2 | 67.0 ± 27.9 | 56.8 ± 27.3 | 72.4 ± 23.6 | 5.22 (− 3.93,14.38) | 8.24 (− 1.73,18.22) |
| No. usable embryos (i.e., frozen or transferred)* | 2.0 ± 1.6 | 3.3 ± 2.9 | 2.2 ± 1.4 | 3.4 ± 1.9 | 0.11 (− 0.79,1.03) | −0.01 (− 0.99,0.98) |
| % Usable embryos/2PN | 76.2 ± 31.6 | 72.7 ± 28.6 | 68.1 ± 33.8 | 67.7 ± 34.0 | − 0.04 (− 0.13,0.06) | − 0.04 (− 0.15,0.06) |
*Values are mean ± SD
+Of total oocytes
++Comparing cycle 2 outcomes, adjusted for age
**Adjusted for age, stimulation protocol, trigger type, hours to retrieval, and insemination type
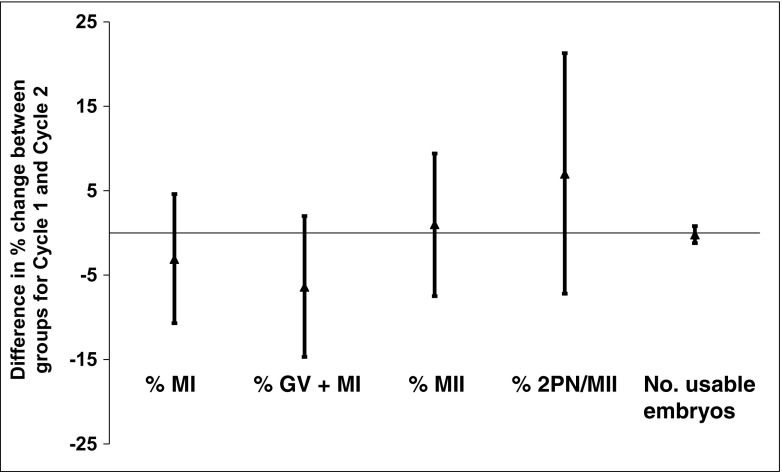
There was a similar increase in %MII oocytes between cycles 1 and 2 for both treated (48.1 ± 15.7 to 68.9 ± 20.3%) and untreated groups (50.5 ± 14.8 to 72.5 ± 21.7%) [linear regression β 1.26 (− 6.7, 8.80)], as well as for %2PN zygotes [treated 56.8 ± 27.3 to 72.4 ± 23.6% vs. untreated 59.0 ± 32.2 to 67.0 ± 27.9%, linear regression β 8.24 (− 1.73, 18.22); Table 3 and Fig. 1]. Similarly, the number of usable embryos in cycle 2 did not differ significantly between the two groups [treated vs. untreated: 3.4 ± 1.9 vs. 3.3 ± 2.9, respectively, linear regression β − 0.01 (− 0.99, 0.98); Table 3]. The incidence of failed fertilization was similar between the treated and untreated groups in cycle 2 [2.5 vs. 7.0%, OR 0.17 (0.02-1.52); Table 4].
Fig. 1.
Difference in percent change of oocyte maturity and embryo usability from cycle 1 to cycle 2 for the treated compared to untreated groups. Compared to the untreated group, negative values indicate that there was a greater decrease in the given outcome from cycle 1 to cycle 2 in the treated group, while positive values indicate a greater increase from cycle 1 to cycle 2
Table 4.
Clinical outcomes of the untreated and treated groups in cycle 2 (n = 198)*
| Untreated group (n = 158) | IVM treated group (n = 40) | Logistic regression OR (95% CI)++ | Logistic regression AOR (95% CI)++*** | |
|---|---|---|---|---|
| No. patients with failed fertilization (% total) | 11 (7.0%) | 1 (2.5%) | 0.36 (0.04-2.86) | 0.17 (0.02-1.52) |
| Implantation rate (#sacs/# transferred)# | 23.1% | 20.3% | 1.14 (0.71–1.83) | 1.22 (0.72–2.09) |
| Cycle outcome | ||||
| No. freeze-all | 8 (5.1%) | 2 (5.0%) | 0.97 (0.20–4.77) | 0.70 (0.10–4.93) |
| No. with transfer | 139 (88.0%) | 37 (92.5%) | 1.65 (0.46–5.90) | 3.26 (0.75–14.24) |
| No. chemical/ectopic | 31 (19.6%) | 5 (12.5%) | 0.58 (0.21–1.61) | 0.59 (0.20–1.75) |
| No. clinical pregnancy (with sac) | 51 (32.3%) | 14 (35.0%) | 1.11 (0.53–2.32) | 1.27 (0.57–2.84) |
| No. to OB care+ | 44 (27.9%) | 12 (30.0%) | 1.08 (0.50–2.32) | 1.30 (0.56–3.03) |
| No. live birth** | 42 (26.8%) | 12 (30.0%) | 1.14 (0.53–2.45) | 1.33 (0.57–3.11) |
*PGD/PGS cycles excluded
#Poisson regression used
+Pregnancies that graduated to OB Care but that have not yet delivered
**Live births, calculated from n = 157
++OR odds ratio, CI confidence interval: comparing outcomes of cycle 2, adjusted for age
***AOR adjusted odds ratio adjusted for age, stimulation protocol, trigger type, hours to retrieval, and insemination type
Day 3 embryo quality
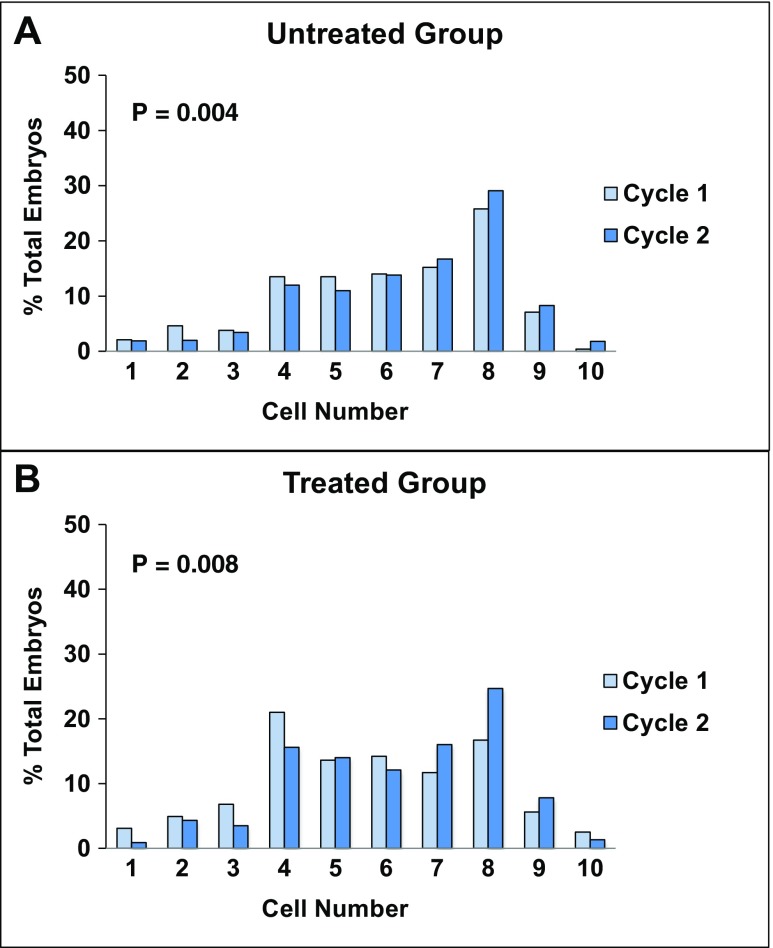
The number of women with at least one good quality D3 embryo in cycle 2 was comparable between the two groups (treated vs. untreated 53.5 vs. 57.1%), as also was the % good quality embryos per woman (treated vs. untreated 23.7 vs. 28.3). While the distribution of embryos by cell number was shifted in favor of higher cleavage stages from cycles 1 to 2 in both groups (treated, p = 0.008; untreated, p = 0.004; Fig. 2), the difference in distribution between groups for cycles 1 and 2 did not reach statistical significance (p = 0.06). On average per woman, the proportion of D3 embryos with ≥ 8 cells increased from 21.5 to 32.2% (p = 0.07) from cycle 1 to cycle 2 in the treated group, vs. from 28.4 to 36.2% from cycle 1 to cycle 2 in the untreated group (p = 0.03).
Fig. 2.
Comparison of distributions of day 3 embryos by cell number between cycles 1 and 2 for untreated and treated (IVM) groups. Distributions for the untreated (unexposed) group are shown in a; those for the treated (IVM) group are shown in b. Embryo distributions in cycle 1 are shown in light blue columns; those in cycle 2 are shown in dark blue columns. Statistically significant differences in cell number distributions from cycle 1 to cycle 2 were observed in both untreated (p = 0.004) and treated (p = 0.008) groups. A trend towards different cell number distributions between the untreated and treated groups was observed in cycle 2 (p = 0.06)
Clinical outcomes
Overall, there were no statistically significant differences in clinical outcomes between the treated and untreated groups in cycle 2. As shown in Table 4, implantation rates [AOR 1.22 (0.72–2.09), clinical pregnancy rates [AOR 1.27 (0.57–2.84)], and live birth rates were similar, with 12/14 (30.0%) live births in the treated group and 42/54 (26.8%) live births in the untreated group [AOR 1.33 (0.57–3.11)].
Discussion
This study was undertaken to investigate whether short-term incubation of COCs in IVM medium to increase mature oocyte yield from metaphase I oocytes improves outcomes for patients with a prior unsuccessful cycle taking their previous proportion of immature oocytes into consideration. Specifically, the study was designed to identify any increases in the proportion of mature oocytes and usable embryo yield after 3–5 h of exposure of COCs to IVM medium, greater than those seen in a matched untreated group. The 3–5-h duration of incubation was selected to model the observed length of progression of human oocytes from metaphase I to metaphase II in vitro. Although we did identify increases in each of these variables in cycles using short-term exposure to IVM medium, the increases were small and none was statistically significantly greater than those observed in our match-paired untreated cohort. These results therefore do not support the hypothesis that short-term exposure to IVM medium using the protocol described here is beneficial for a population of women with previously unsuccessful cycles notable for a high proportion of immature oocytes.
Prior data indicate that IVM-matured MI oocytes can yield usable embryos of improved quality over simply injecting arrested MI oocytes [2]. This finding is not surprising given that oocytes must achieve cytoplasmic and nuclear maturity with their progression to metaphase II before fertilization normally occurs. However, IVM medium is expensive and possesses a short half-life, making this procedure not only more time- and skill-intensive, but also more expensive for the patient [25]. It remains unclear whether routine use of short-term IVM in stimulated cycles offers an improvement in clinical outcomes compared to conventional IVF/ICSI, thereby warranting these increased costs.
We observed that in a cohort of women matched on %MI and %MII oocytes in their initial cycle, the use of short-term IVM in the subsequent cycle was not associated with any significant changes in the proportion of MI oocytes, MII oocytes, or 2PNs compared to the untreated control group. The increase in the number of usable embryos from cycle 1 to 2 was also similar between groups, likely attributable to changes in clinical or laboratory protocols over time. Moreover, there were no significant differences in embryologic outcomes after additionally adjusting for follicle size < 16 mm (data not shown). Of note, the mean percentage of immature oocytes in cycle 1 of the treated group was 48.2%, far above the typical 15–30% seen in oocyte cohorts after ovarian stimulation. Therefore, our results may only be applicable to populations with an abnormally high proportion of immature oocytes.
There were no differences in the clinical pregnancy and live birth rates between the treated and untreated groups in cycle 2, though both variables improved, as anticipated, after a failure to conceive in cycle 1. The lower implantation rate in the treated group is consistent with prior findings for MI oocytes exposed to rescue IVM [4] and may be explained by a study by Walls and colleagues that in vitro matured oocytes are less likely to reach blastocyst stage [26], although this was a small study using different IVM protocols from those used in our study. Interestingly, despite prior reports of lower fertilization rates in in vitro matured oocytes [2, 3], threefold fewer women in the treated group had failed fertilization in cycle 2 (2.3 vs. 6.8%). Nevertheless, this difference did not reach statistical significance, likely due to the relatively small number of patients in our study population.
At the embryo cohort level, our short-term exposure to IVM medium was associated with a significant shift in the cell number distribution of D3 embryos (p = 0.008), with an increase in ≥ 8 cell embryos (24.7 to 33.4% from cycle 1 to 2, p = 0.05), suggesting a positive impact on embryo development. Although changes in stimulation protocols may have also influenced these results, this is an important finding that warrants further investigation given previous reports that embryo quality may be reduced with short-term, as well as extended, IVM [4, 9]. Ultimately, however, women had similar proportions of good quality embryos in cycle 2 regardless of IVM status.
This study has several strengths. First, it examines both embryologic and clinical outcomes of short-term IVM exposure in a matched cohort of patients with similar baseline percentages of MI and MII oocytes. Thus, any association between the proportion of MII oocytes and overall cohort maturity was controlled. Second, cycle 1 served as an internal control for each patient, thereby enabling analysis and comparison of any inter-cycle improvement in outcomes for both the untreated and treated group. This design thus facilitated distinction between any benefits attributable to the IVM treatment per se in cycle 2, compared to improvements resulting from any change in ovarian stimulation regimen, based on response in cycle 1. Indeed, the improvements occurred despite relatively similar total numbers of oocytes retrieved between the cycles, an outcome that can vary with gonadotropin dosing [27]. Also of note, age, stimulation protocol, trigger type, and hours from trigger to retrieval were controlled in the analyses as potential confounders that can impact oocyte maturity. Likewise, insemination type was controlled as this may influence fertilization rates. Third, we used a standardized short-term IVM protocol: all COCs in the treated group were consistently placed in IVM (SAGE) medium for 3–5 h prior to stripping and IVF/ICSI. This protocol is unique from many previous IVM studies in which the cumulus cells were removed before exposure to IVM medium [2, 3, 9, 15]. By keeping the COCs intact during culture, we maintained the normal physiological relationship between the oocyte and its surrounding somatic cells, which is known to be critical for acquisition of oocyte developmental competency [10, 11, 28].
Our study also has several limitations. Since IVM is not routinely used at our institution, a relatively small number of IV cycles were available for our study inclusion, limiting the power of this study. The data were also drawn from a wide range of years during which changes in stimulation protocols occurred that could not be controlled for in our analyses, although every effort was made to match cycles by date of retrieval. Furthermore, there were no significant differences in average year of treatment between the untreated and treated groups (data not shown). We included both conventional IVF and ICSI cycles, which were controlled for in our analyses; however, our embryo quality and clinical findings are not specific to one fertilization method. Other than accounting for age, ovarian reserve was not controlled, and on average, the AMH levels were lower in the treated group, though still well within normal range. Moreover, we could not account for provider bias in referring patients for short-term IVM, although our rigorous matching criteria ensured that the proportion of immature oocytes in cycle 1 was not significantly different between groups. Finally, despite similar demographic characteristics and ovarian stimulation protocols for the treated and untreated groups in each cycle, underlying infertility diagnoses and resulting prognosis could not be controlled.
In conclusion, our findings demonstrate that in a population of women with a prior unsuccessful IVF cycle and an elevated proportion of immature oocytes despite ovarian stimulation and use of an ovulatory trigger, short-term exposure of COCs to IVM medium does not appear to be beneficial in the subsequent cycle, based on the similar yield of mature oocytes, zygotes, and good quality D3 embryos compared to conventional handling of COCs in our program. These results therefore do not support use of short-term exposure of COCs to IVM medium in this group of patients. Further work is required to examine application of this “rescue IVM” approach in a larger study population, as well as to determine whether there is a subset of patients who may have improved clinical outcomes with this intervention.
Acknowledgements
The authors acknowledge the Brigham and Women’s Hospital Embryology team for their assistance in data retrieval.
Compliance with ethical standards
This retrospective study was approved by the Partners’ Healthcare Institutional Review Board.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
C. R. Sacha, Email: csacha@partners.org
D. J. Kaser, Email: dkaser@rmanj.com
L. V. Farland, Email: lfarland@hsph.harvard.edu
S. Srouji, Email: ssrouji@partners.org
S. A. Missmer, Email: stacey.missmer@hc.msu.edu
C. Racowsky, Phone: 617-732-5455, Email: cracowsky@bwh.harvard.edu
References
- 1.Edirisinghe W, Junk S, Matson P, Yovich J. Birth from cryopreserved embryos following in-vitro maturation of oocytes and intracytoplasmic sperm injection. Hum Reprod. 1997;12(5):1056–1058. doi: 10.1093/humrep/12.5.1056. [DOI] [PubMed] [Google Scholar]
- 2.Strassburger D, Friedler S, Raziel A, Kasterstein E, Schachter M, Ron-El R. The outcome of ICSI of immature MI oocytes and rescued in vitro matured MII oocytes. Hum Reprod. 2004;19(7):1587–1590. doi: 10.1093/humrep/deh236. [DOI] [PubMed] [Google Scholar]
- 3.De Vos A, Van de Velde H, Joris H, Van Steirteghem A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum Reprod. 1999;14(7):1859–1863. doi: 10.1093/humrep/14.7.1859. [DOI] [PubMed] [Google Scholar]
- 4.Vanhoutte L, De Sutter P, Van der Elst J, Dhont M. Clinical benefit of metaphase I oocytes. Reprod Biol Endocrinol. 2005;3:71. doi: 10.1186/1477-7827-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vos M, Smitz J, Thompson JG, Gilchrist RB. The definition of IVM is clear—variations need defining. Hum Reprod. 2016;31(11):2411–2415. doi: 10.1093/humrep/dew208. [DOI] [PubMed] [Google Scholar]
- 6.Kim B, Lee S, Kim K, Han C, Kim J. In vitro maturation, fertilization, and development of human germinal vesicle oocytes collected from stimulated cycles. Fertil Steril. 2000;74(6):1153–1158. doi: 10.1016/S0015-0282(00)01617-4. [DOI] [PubMed] [Google Scholar]
- 7.Chang E, Song H, Lee D, Lee W, Yoon T. In vitro maturation of human oocytes: its role in infertility treatment and new possibilities. Clin Exp Reprod Med. 2014;41(2):41–46. doi: 10.5653/cerm.2014.41.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko DS, Lee SH, Park DW, Yang KM, Lim CK. Pregnancy and fertilization potential of immature oocytes retrieved in intracytoplasmic sperm injection cycles. Clin Exp Reprod Med. 2015;42(3):118–125. doi: 10.5653/cerm.2015.42.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichman D, Politch J, Ginsburg E, Racowsky C. Extended in vitro maturation of immature oocytes from stimulated cycles: an analysis of fertilization potential, embryo development, and reproductive outcomes. J Assist Reprod Genet. 2010;27:347–356. doi: 10.1007/s10815-010-9416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21(4):427–454. doi: 10.1093/humupd/dmv011. [DOI] [PubMed] [Google Scholar]
- 11.Coticchio G, Dal-Canto M, Guglielmo MC, Mignini-Renzini M, Fadini R. Human oocyte maturation in vitro. Int J Dev Biol. 2012;56(10–12):909–918. doi: 10.1387/ijdb.120135gv. [DOI] [PubMed] [Google Scholar]
- 12.Brown HM, Dunning KR, Sutton-McDowall M, Gilchrist RB, Thompson JG, Russell DL. Failure to launch: aberrant cumulus gene expression during oocyte in vitro maturation. Reproduction. 2017;153:R109–R120. doi: 10.1530/REP-16-0426. [DOI] [PubMed] [Google Scholar]
- 13.Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- 14.Gilchrist RB, Luciano AM, Richani D, Zeng HT, Wang X, Vos MD, et al. Oocyte maturation and quality: role of cyclic nucleotides. Reproduction. 2016;152(5):R143–R157. doi: 10.1530/REP-15-0606. [DOI] [PubMed] [Google Scholar]
- 15.Strassburger D, Goldstein A, Friedler S, Raziel A, Kasterstein E, Mashevich M, et al. The cytogenetic constitution of embryos derived from immature (metaphase I) oocytes obtained after ovarian hyperstimulation. Fertil Steril. 2010;94(3):971–978. doi: 10.1016/j.fertnstert.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Walls M, Hart R, Keelan J, Ryan J. Structural and morphologic differences in human oocytes after in vitro maturation compared with standard in vitro fertilization. Fertil Steril. 2016;106(6):1392–1398. doi: 10.1016/j.fertnstert.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Li Y, Gao X, Yan J, Chen Z. Changes in the distribution of mitochondria before and after in vitro maturation of human oocytes and the effect of in vitro maturation on mitochondria distribution. Fertil Steril. 2010;93(5):1550–1555. doi: 10.1016/j.fertnstert.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Jones GM, Cram DS, Song B, Magli MC, Gianaroli L, Lacham-Kaplan O, et al. Gene expression profiling of human oocytes following in vivo or in vitro maturation. Hum Reprod. 2008;23(5):1138–1144. doi: 10.1093/humrep/den085. [DOI] [PubMed] [Google Scholar]
- 19.Racowsky C, Ohno-Machado L, Kim J, Biggers J. Is there an advantage in scoring early embryos on more than one day? Hum Reprod. 2009;24(9):2104–2113. doi: 10.1093/humrep/dep198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machtinger R, Bormann C, Ginsburg E, Racowsky C. Is the presence of a non-cleaved embryo on day 3 associated with poorer quality of the remaining embryos in the cohort? J Assist Reprod Genet. 2015;32(5):677–683. doi: 10.1007/s10815-015-0455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skiadas C, Jackson K, Racowsky C. Early compaction on day 3 may be associated with increased implantation potential. Fertil Steril. 2006;86(5):1386–1391. doi: 10.1016/j.fertnstert.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Machtinger R, Politch J, Hornstein M, Ginsburg E, Racowsky C. A giant oocyte in a cohort of retrieved oocytes: does it have any effect on the in vitro fertilization cycle outcome? Fertil Steril. 2011;95(2):573–576. doi: 10.1016/j.fertnstert.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 23.Reichman D, Jackson K, Racowsky C. Incidence and development of zygotes exhibiting abnormal pronuclear disposition after identification of two pronuclei at the fertilization check. Fertil Steril. 2010;94(3):965–970. doi: 10.1016/j.fertnstert.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Racowsky C, Combelles C, Nureddin A, Pan Y, Finn A, Miles L, et al. Day 3 and day 5 morphological predictors of embryo viability. Reprod BioMed Online. 2003;6(3):323–331. doi: 10.1016/S1472-6483(10)61852-4. [DOI] [PubMed] [Google Scholar]
- 25.Pongsuthirak P, Songveeratham S, Vutyavanich T. Comparison of blastocyst and Sage media for in vitro maturation of human immature oocytes. Reprod Sci. 2015;22(3):343–346. doi: 10.1177/1933719114542027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walls M, Ryan J, Keelan J, Hart R. In vitro maturation is associated with increased early embryo arrest without impairing morphokinetic development of useable embryos progressing to blastocysts. Hum Reprod. 2015;30(8):1842–1849. doi: 10.1093/humrep/dev125. [DOI] [PubMed] [Google Scholar]
- 27.Eppsteiner E, Sparks A, Liu D, Van Voorhis B. Change in oocyte yield in repeated in vitro fertilization cycles: effect of ovarian reserve. Fertil Steril. 2014;101(2):399–402. doi: 10.1016/j.fertnstert.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 28.Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturaiton in in-vitro matured human oocytes. Hum Reprod. 2002;17(4):1006–1016. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]