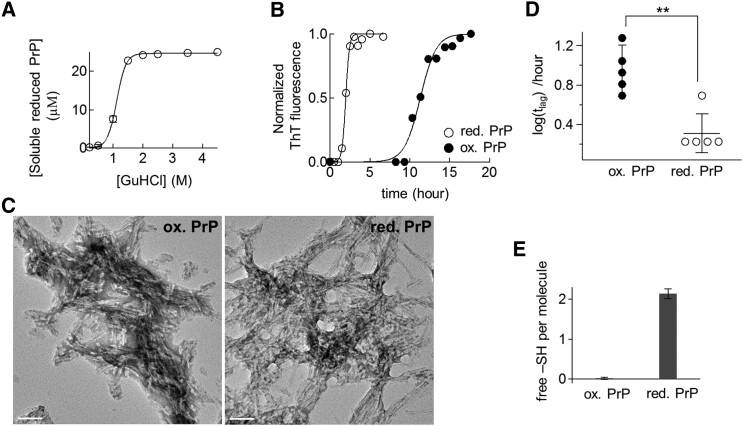
Figure 2.
(A) Solubilities of PrP at various concentrations of GuHCl. Reduced PrP (25 μM) was incubated for 1 h at 37°C in the presence of various concentrations of GuHCl and centrifuged at 62,900 × g for 30 min. The concentration of soluble protein was measured using UV absorbance at 280 nm. (B) Representative time courses of amyloid formation from oxidized (solid circles) and reduced PrP (open circles) at a GuHCl concentration of 2 M. ThT-fluorescence was normalized to its maximum value (346 ± 96 and 740 ± 380 a.u. for oxidized and reduced proteins, respectively). The solid lines represent best fits to a sigmoidal function. (C) Transmission electron micrographs of the amyloid fibrils generated from oxidized (left) or reduced PrP (right). Scale bars, 100 nm. (D) Lag time of amyloid formation derived from Fig. 2B (∗∗p < 0.005, unpaired two-tailed t-test). (E) The number of free –SH group detected in a protein monomer dissociated from amyloid fibrils.