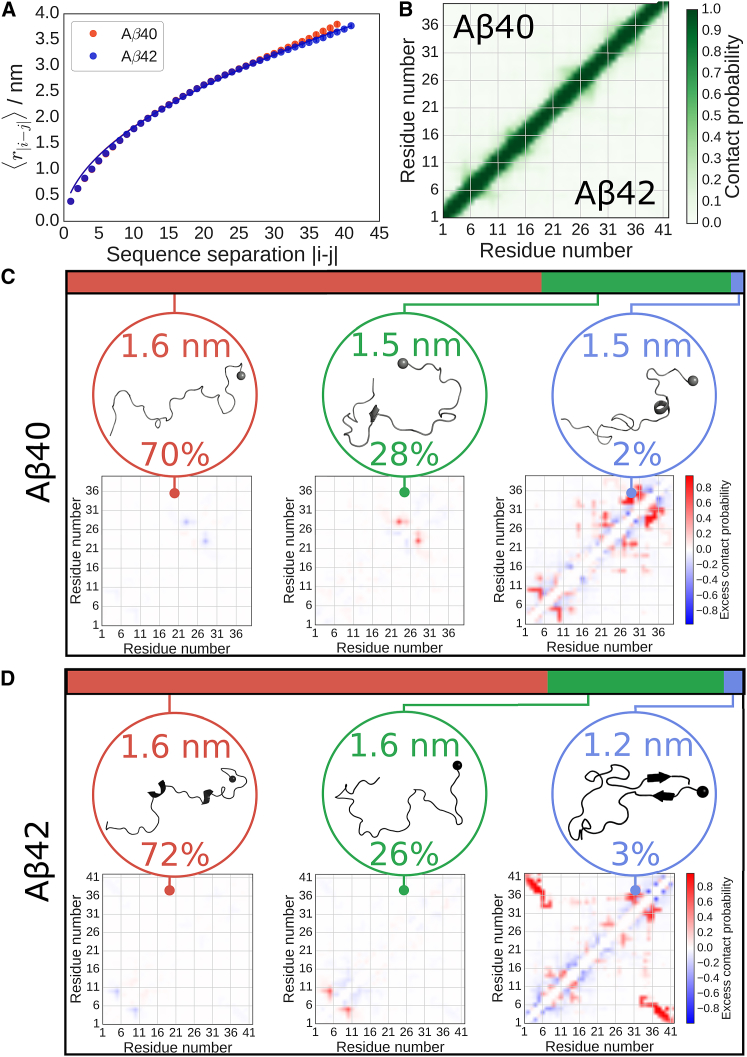
Figure 7.
Conformational ensembles of Aβ40 and Aβ42. (A) Internal Cα atom distance scalings for each peptide, with data shown as circles, and fits to a power law as described in the Supporting Material as solid lines, giving scaling exponents of ν = 0.5197 ± 0.0006 and 0.5180 ± 0.0004, respectively, for Aβ40 and Aβ42. Note that the similarity in peptide behaviors means that the fitted curve for Aβ40 overlaps with that of Aβ42. (B) Simulation-averaged intramolecular contact maps show no significant secondary structure for either peptide, with Aβ40 shown in the upper half and Aβ42 in the lower. (C) Major clusters for the structural ensemble of Aβ40. The top colored bar denotes the observed statistical weights of each subpopulation, for which the most representative structure (with the N-terminus denoted by a sphere) and average intramolecular contact map relative to the ensemble-averaged contact map are shown. Blue contacts are those populated less frequently than the average, whereas red are those populated more frequently. The average radius of gyration and population for each cluster are shown accompanying the structure. The first cluster is random coil, the second populates very local contacts, and the last shows structure within the termini. (D) Major clusters for the structural ensemble of Aβ42. Similarly to the Aβ40 ensemble, the first cluster is random coil and the second populates very local contacts, whereas the last shows the structure formation between the termini. To see this figure in color, go online.