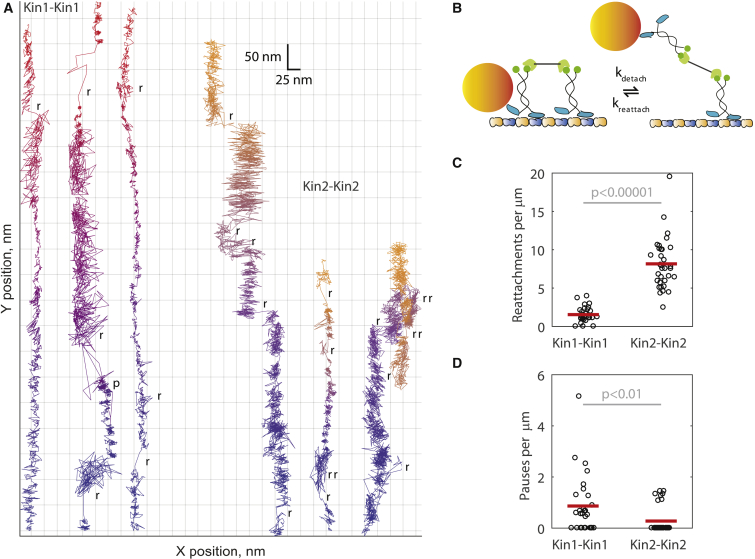
Figure 3.
High resolution single-molecule tracking reveals that kinesin-2 reattaches more often and pauses less often than kinesin-1. (A) Example 1000 frames per second traces of Kin1-Kin1 (blue-red) and Kin2-Kin2 (blue-yellow) pairs. A single motor domain of one motor was tagged with a 30-nm gold nanoparticle (shown in diagram in B), and the particle position imaged by dark-field total internal reflection microscopy (see Materials and Methods). Time information is encoded in color (see Fig. S4 for the same data displayed as position versus time). Of note are abrupt positional changes that intersperse normal stepping, indicating reattachment events, and areas of high versus low variance, indicating whether labeled motors are engaged with the microtubule. Scored rebinding events (r) and pauses (p) are highlighted on each trace. (C) Kin2-Kin2 pairs reattach more often than Kin1-Kin1 pairs. Reattachments were scored as jumps >40 nm in the Y position (parallel to the microtubule) or >15 nm in the X position (sidesteps). Kin1-Kin1 pairs reattached 1.54 ± 0.19 times, whereas Kin2-Kin2 pairs reattached 8.16 ± 0.58 times per micron traveled (mean ± SE; N = 29 and N = 33 traces, respectively, with plot showing one point per trace and mean values as red bars). A 2-sample t-test indicated that the difference in reattachment frequency was significant (p < 0.00001). (D) Kin1-Kin1 pairs pause more often than Kin2-Kin2 pairs. Pauses were scored as instances of no positional change lasting longer than 10 step durations (137 ms for kinesin-1 and 410 ms for kinesin-2). Kin1-Kin1 pairs paused 0.86 ± 0.21 times per micron traveled (mean ± SE, N = 29 traces), whereas Kin2-Kin2 paused 0.28 ± 0.09 times per micron traveled (mean ± SE, N = 33 traces). A Mann-Whitney U-test indicated that the difference in pausing frequency was significant (p < 0.01).